Anemia is a decrease in hematocrit, hemoglobin, and/or RBC concentration that results in decreased blood-oxygen carrying capacity, which can lead to nonspecific clinical signs (eg, lethargy, exercise intolerance, depression).1 Anemia is generally classified as regenerative or nonregenerative based on bone marrow response, with an appropriate response indicating regenerative anemia and persistent absence of an appropriate response indicating nonregenerative anemia.1
Regenerative anemia is suspected if anemia is caused by RBC loss from hemorrhage or hemolysis.1 Regeneration is typically evident on CBC (reticulocytosis) within 3 to 5 days of onset of hemorrhage or hemolysis.2 Preregenerative anemia may be assumed if the patient is assessed before 3 days have elapsed and clinical evidence suggests bone marrow will respond. Absence of reticulocytosis within 5 to 7 days of onset of anemia indicates nonregenerative anemia because sufficient time has passed for a regenerative bone marrow response.1
Determining the cause of nonregenerative anemia can be challenging because there are numerous potential etiologies. Following are the top 5 pathologies that can result in nonregenerative anemia, according to the authors.
1. Anemia of Inflammation
Anemia of inflammation (formerly, anemia of chronic disease) is a common cause of mild to moderate nonregenerative anemia typically characterized by normocytic, normochromic RBC morphology with little to no associated poikilocytoses.3,4 Any chronic disease that causes a prolonged systemic inflammatory response may lead to nonregenerative anemia.4 Pathogenesis of anemia of inflammation can be attributed to impaired iron metabolism, impaired bone marrow function, or reduced RBC lifespan.5
With unimpaired iron metabolism, old erythrocytes are phagocytized by tissue macrophages. Iron is then transported out of the macrophages through a membrane-bound protein (ie, ferroportin) to be reused for erythropoiesis.5 Transport via ferroportin is essential for iron to exit the macrophage.5 Hepcidin, an acute-phase protein that increases in response to circulating inflammatory cytokines (most notably interleukin-6 and interleukin-1), binds to and causes internalization and degradation of ferroportin, trapping iron inside tissue macrophages3; therefore, iron is inaccessible with anemia of inflammation despite normal to increased total body iron stores.
Although impaired iron metabolism initiates anemia of inflammation, recent studies in dogs and mice have shown hepcidin concentration is increased only in the initial stages of inflammation, suggesting other factors may play a role.5 Studies in rats and humans have shown cytokines can inhibit production of erythropoietin (EPO; a hormone produced by peritubular interstitial tissue in the kidneys) as well as EPO-mediated differentiation and proliferation of erythroid precursors.3 Little research on serum EPO concentrations in dogs is available. Studies in cats suggest blunting of the erythrocyte response to EPO and/or inhibition of erythrocyte progenitor differentiation may contribute more than serum EPO concentration to maintain the anemic state.3,5
Studies in dogs and cats with experimentally induced abscesses indicate chronic systemic inflammation can reduce RBC lifespan.3 Premature removal of RBCs via macrophagic erythrophagocytosis results from inflammatory cytokine-triggered macrophage activation and immunoglobulin coating of RBCs.5
2. Anemia of Chronic Kidney Disease
Anemia of chronic kidney disease (CKD; ie, anemia of renal disease) is a common cause of nonregenerative anemia typically characterized by a normocytic, normochromic RBC morphology and can be mild, moderate, or severe based on the stage of CKD.6-8 Prevalence increases with patient age, especially in cats (up to 80% in geriatric cats).9 EPO deficiency is the main etiology of anemia of CKD.7
In healthy patients with inadequate tissue oxygenation, hypoxia-inducible factor-1 alpha (HIF-1 alpha) stimulates upregulation of EPO production to increase RBC concentration, which increases oxygen-carrying capacity and returns the body to a nonhypoxic state.6 Proper oxygenation leads to degradation of HIF-1 alpha and decreased EPO production and release.6 CKD results in progressive destruction of peritubular interstitial cells, leading to inadequate EPO production, despite increased HIF-1 alpha.6 As CKD progresses, RBC production decreases, with further loss of renal function and destruction of EPO-producing tissue in the kidneys.6,8 A study in dogs showed severe decreases in hematocrit and a high percentage of dogs with no evidence of regeneration (<60,000 reticulocytes/µL) as CKD progressed toward International Renal Interest Society stage 4.8
Other factors (eg, impaired renal clearance, chronic inflammation) also contribute to development of anemia in patients with renal disease.8 The kidneys are unable to effectively filter and remove metabolites, accumulation of which can lead to uremia.10 In dogs and cats, uremia can increase RBC fragility, which may result in premature or increased clearance of RBCs.6,7 Uremia is also hypothesized to deleteriously affect coagulation, which may contribute to anemia via blood loss through uremia-induced alimentary tract ulcers.6,7
3. Hormone-Associated Anemia
Endocrinopathies, especially hypothyroidism and hypoadrenocorticism, are hormonal disorders that can cause nonregenerative anemia (typically in dogs) characterized as mild, with normocytic, normochromic RBC morphology.1
Thyroid hormone has many important roles in the body, including maintenance of normal hematocrit.11,12 Patients with hypothyroidism have a lower metabolic rate (compared with healthy patients) that results in decreased energy consumption and oxygen requirements.11,13 Dogs with hypothyroidism-associated nonregenerative anemia typically have decreased EPO concentrations, which may indicate an adaptation to produce less EPO in response to anemia due to the decreased metabolic rate and decreased physiologic requirement for oxygen.11 Alternatively, some studies hypothesize that decreased erythrocyte production may be due to decreased EPO production, decreased response to EPO, and decreased direct effect of EPO on early hemopoietic pluripotent stem cells as a result of decreased thyroid hormone.14 More research is needed to clearly define the mechanisms involved.
The mechanism of nonregenerative anemia in hypoadrenocorticism is not fully understood. Normal production of corticosteroids and androgens likely affects stimulation of erythropoiesis in bone marrow; conversely, lack of these hormones likely decreases EPO production.15 Ongoing fluid losses may mask the severity of anemia but can be seen following fluid therapy.15 Concurrent alimentary tract hemorrhage may also exacerbate anemia.15
4. Immune-Mediated Nonregenerative Anemia
Immune-mediated nonregenerative anemias (ie, pure red cell aplasia [PRCA], precursor immune-mediated anemia [PIMA]) result in persistent anemia with an absence of reticulocytosis.16 Unlike immune-mediated hemolytic anemia (IMHA), which is a well-understood disorder characterized by immune-mediated targeting of mature RBCs that results in regenerative anemia, the mechanisms of action and pathogeneses of PRCA and PIMA are not clearly defined. PRCA and PIMA, however, are categorized as immune-mediated disorders because current evidence supports idiopathic immune-mediated targeting of erythroid precursors (Figure 1) and these patients are typically responsive to immunosuppressive therapy and relapse following discontinuation of therapy.16-18
PRCA and PIMA typically result in severe, normocytic, normochromic, nonregenerative anemias. Morphologic changes of RBCs are not expected on blood smear examination in patients with PRCA. Conversely, in patients with PIMA, macrocytosis and elliptocytosis (ovalocytes) are common16; however, absence of these RBC morphologies does not rule out PIMA. With PRCA, megakaryocytes and granulocytic precursors are not affected; platelet and neutrophil concentrations are thus typically within the reference interval—a feature that differentiates PRCA from aplastic anemia. In some cases of PIMA, other concurrent cytopenias (eg, neutropenia, thrombocytopenia) have been reported.16
In healthy patients, the first stage of committed erythropoiesis is the progenitor cell burst-forming unit-erythroid (BFU-E), which differentiates into the colony-forming unit-erythroid (CFU-E). CFU-E produces additional precursor cells, with each CFU-E yielding 8 to 32 mature RBCs.19 In patients with immune-mediated anemias, a specific erythrocyte stage (or stages) is targeted for destruction, and the disease is classified based on the stage or group of stages targeted.16 In patients with IMHA, mature RBCs are targeted by the immune system and destroyed.18 In patients with PRCA, BFU-E, CFU-E, or early rubriblasts are destroyed, resulting in an absence of an identifiable erythroid lineage on routine bone marrow examination. PIMA results from targeting of any stage after CFU-E/early rubriblast and before mature RBCs, from rubriblast to reticulocyte.16
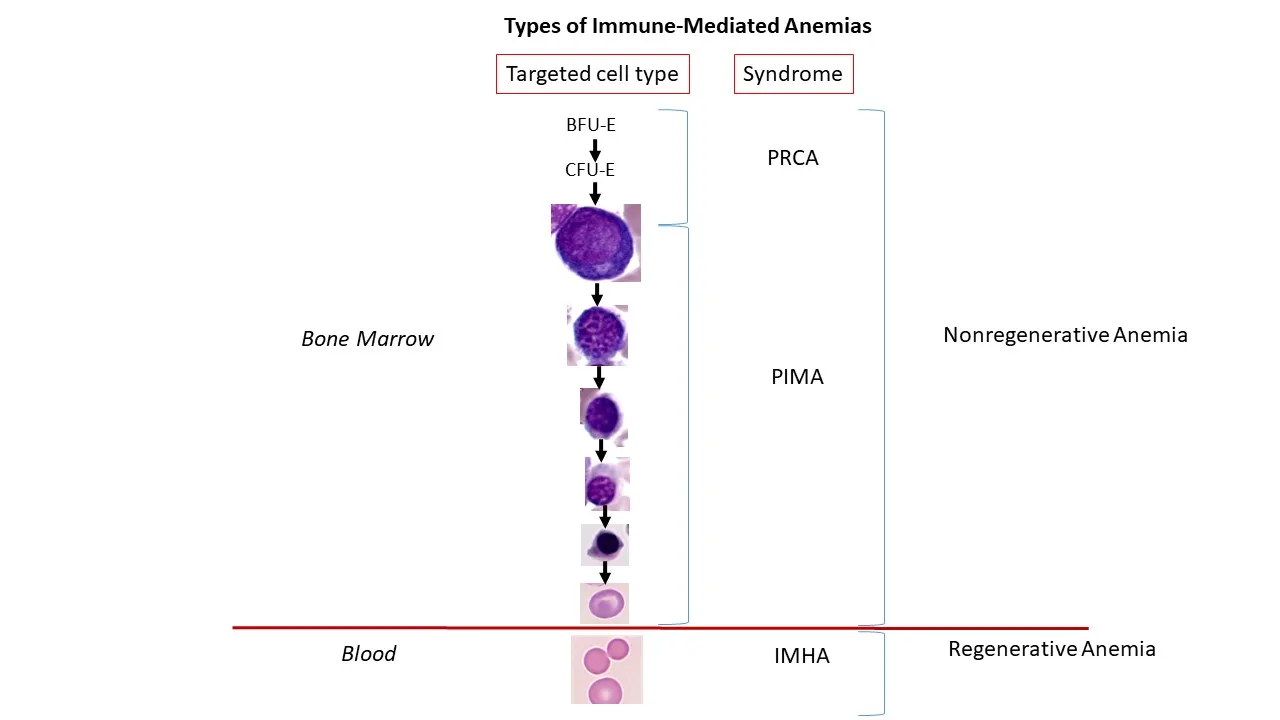
This illustration depicts the stages of orderly erythropoiesis and types of immune-mediated anemias that develop depending on the erythrocyte maturation cell-stage targeted for destruction. In cases of IMHA, mature RBCs are targeted by the immune system and destroyed.18 In cases of PRCA, BFU-E, CFU-E, or early rubriblasts are destroyed. PIMA targets stages after CFU-E/early rubriblast and before mature RBCs.16
Basal erythropoiesis replaces naturally dying RBCs in healthy patients. In patients with PIMA or PRCA, immune-mediated destruction of erythrocyte precursors and progenitors, respectively, leads to an inability to replace naturally dying RBCs. This ineffective erythropoiesis causes a gradual decrease in hematocrit, and anemia develops slowly, giving the body time to adapt to a state of decreased oxygen availability.17,18,20 These anemias are thus typically severe by the time a patient is presented with nonspecific clinical signs (eg, lethargy, anorexia, pale mucous membranes).17,18
Bone marrow evaluation is required for definitive diagnosis of PIMA and PRCA. With PIMA, a bone marrow finding of ineffective erythropoiesis is characterized by an expanded precursor population (erythroid hyperplasia) up to the cell stage being targeted and removed (often referred to as maturation arrest).16 With PRCA, bone marrow examination reveals rare to no erythroid progenitors.16
5. Myelophthisic Anemia (Space-Occupying Lesions)
Myelophthisis refers to any condition that results in ineffective production of normal cell lines in the bone marrow caused by replacement of normal marrow tissue (or environment) with abnormal tissue.21 Replacement of bone marrow with abnormal tissue leads to bone marrow failure and inability to conduct normal hematopoiesis. Patients are typically presented with a combination of nonregenerative anemia, neutropenia, and/or thrombocytopenia.22 Myelophthisic anemia is most commonly seen with primary or secondary infiltrative neoplastic disorders, myelofibrosis, or bone marrow granulomas caused by infectious organisms.21,23-25 Anemia may be the last cytopenia to develop due to the long lifespan of erythrocytes compared with thrombocytes and granulocytes.26
Although any neoplasm that proliferates within bone marrow tissue can potentially cause myelophthisic anemia, lymphoid neoplasia is a common cause of myelophthisis.25 These patients are often presented with an extensive proliferation of the neoplastic cell line that has obliterated the normal structure and function of bone marrow tissue.27 Thrombocytopenia and leukopenia can help differentiate myelophthisis as the cause of nonregenerative anemia from paraneoplastic nonregenerative anemia caused by chronic inflammation or neoplastic infiltration with destruction of the kidneys25; however, bone marrow examination is necessary for definitive diagnosis of myelophthisis.
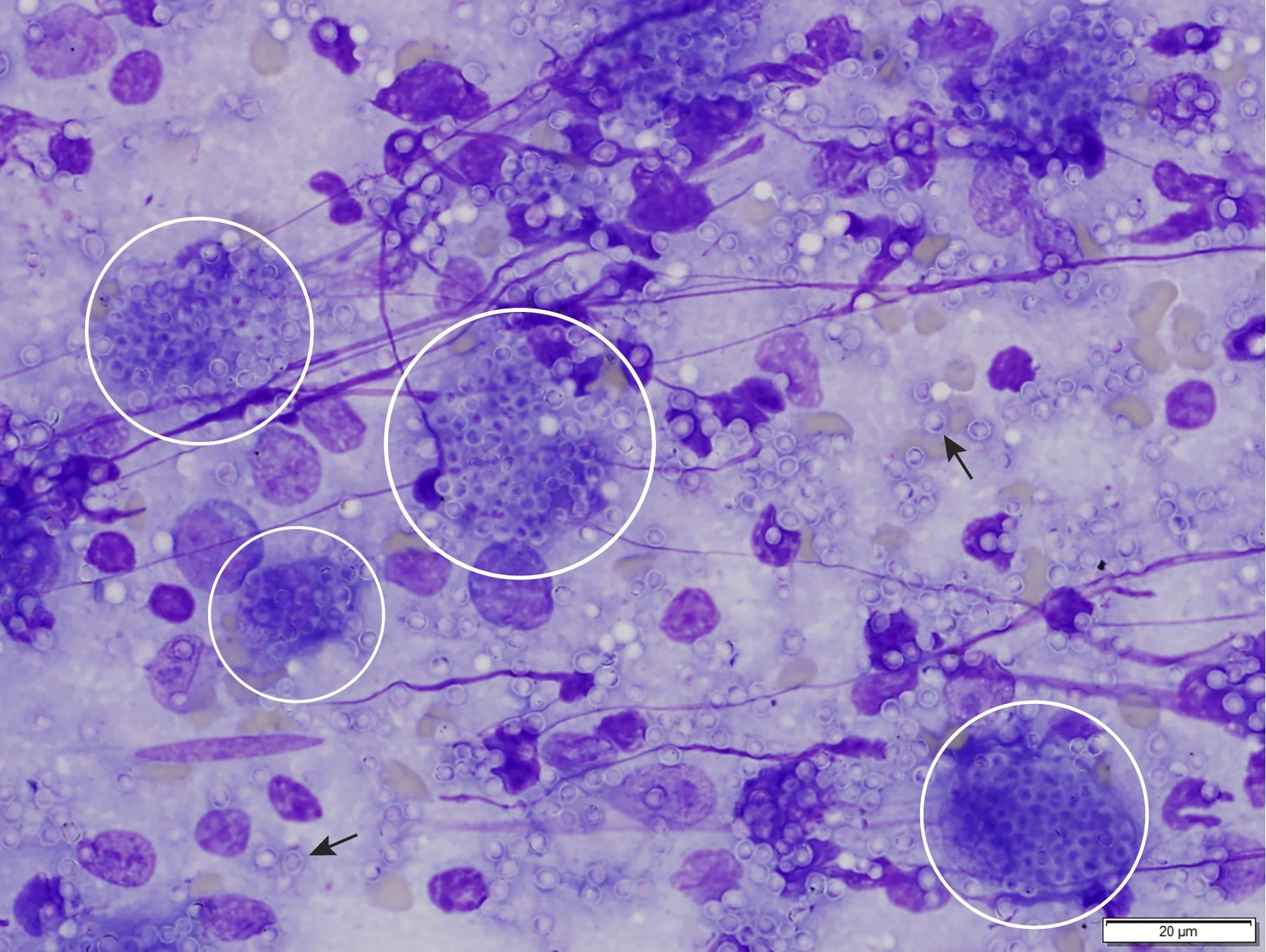
Granuloma formation has been reported with many diseases, including bacterial, rickettsial, fungal, parasitic, and viral infections.23 In these cases, disseminated infectious agents (eg, Leishmania spp, Histoplasma spp; Figure 2) may incite granulomatous inflammation and granuloma formation within the bone marrow, eventually resulting in myelophthisis and bone marrow failure.23,28

Bone marrow aspirate from a cat with myelophthisic anemia and pancytopenia secondary to granulomatous disease due to histoplasmosis. Numerous yeast organisms can be seen extracellularly (arrows) and intracellularly within macrophages (circles), measuring 2 to 4 µm in diameter and with a thin outer halo with eccentrically placed, basophilic, crescent-shaped nuclei. Organisms are consistent with Histoplasma capsulatum. Modified Wright’s stain, 1000× magnification; scale bar = 20 µm
With myelofibrosis, proliferation of fibroblasts, collagen, or reticulin fibers in the hematopoietic space is commonly associated with moderate to severe nonregenerative anemia.29 Primary myelofibrosis—a clonal, myeloproliferative disease—is rarely confirmed in dogs and cats, but secondary myelofibrosis is relatively common.24 The underlying pathogenesis of secondary myelofibrosis is not well understood but may be caused by widespread bone marrow damage with subsequent formation of scar-like tissue.21,29 Microvasculature injury can result in severe ischemia that causes myelonecrosis that may lead to myelofibrosis, especially with chronic disease.30 Patients with myelofibrosis are usually presented with moderate to severe nonregenerative anemia with or without thrombocytopenia and leukopenia (less frequent). Myelofibrosis is usually reversible if the underlying condition is removed or treated.29 Causes of myelofibrosis include IMHA, acute leukemia or lymphoma, and myelotoxic treatment in dogs and IMHA, CKD, FIP, and acute myeloid leukemia in cats.29
Conclusion
Confirmed cases of anemia should be determined to be regenerative (adequate reticulocytosis is present), preregenerative (insufficient time has elapsed for a visible bone marrow response), or nonregenerative (lack of reticulocytosis after anemia has been present 5-7 days). Although nonregenerative anemia has many causes, anemia of inflammation is the most common cause in cats and dogs and should be ruled out first, especially in cases of mild anemia.