Do We Have a New Standard of Care for Acute Onset Pancreatitis in Dogs?
Jörg M. Steiner, MedVet, DrMedVet, PhD, DACVIM, DECVIM-CA, AGAF, Texas A&M University

Sponsored by Ceva Animal Health, LLC
Acute onset pancreatitis (AP) is commonly encountered in dogs and is associated with significant morbidity and even mortality.1 Diagnosis can be challenging and involves the integration of clinical signs, imaging, and clinical pathology, including the measurement of pancreatic lipase in a serum sample.2 However, managing AP in dogs can be even more challenging than achieving a diagnosis.3
The Previous Standard of Care
If identified, specific causes of AP such as hypertriglyceridemia and hypercalcemia should be managed appropriately; however, in many dogs, an underlying cause cannot be identified.4
Fluid therapy is essential to maintain pancreatic microperfusion and hydration status. Because mild dehydration can be difficult to assess, most dogs with AP should be treated with IV fluids, paying close attention to fluid needs and avoiding overhydration while providing enough fluids to compensate for dehydration and ongoing losses.5
AP in dogs can be associated with multiple complications, including single-organ failure (most commonly acute kidney injury, hepatic failure, and acute respiratory failure), ileus, myocarditis, disseminated intravascular coagulation, and multiple organ failure.6 Close monitoring for these potential complications and early intervention are imperative.
Enteral nutritional support should be provided as early as possible, either by offering food for oral intake or by use of a feeding tube (eg, nasogastric tube, esophagostomy tube, gastrostomy tube).7 Jejunostomy tubes or parenteral feeding are rarely indicated. Although there is little evidence on which diets may offer benefits, the author of this paper prefers to feed these patients a diet with a low-fat content (typically <20 g of fat per 1,000 kcal). Antiemetics (ie, maropitant, ondansetron, or a combination of both) and appetite stimulants (ie, capromorelin) can be essential in providing enteral nutrition.
In one study, only 58% of dogs with AP were reported to have abdominal pain,8 whereas in humans with pancreatitis, abdominal discomfort is considered a key clinical sign.9 The author believes that analgesics (excluding NSAIDs) should be provided to any dog presented with AP until there is convincing evidence that abdominal discomfort is absent.6 In many dogs with AP, the presence of abdominal pain typically only becomes apparent when analgesic drugs are provided and the patient shows improvement in attitude, appetite, and/or playfulness.5
A host of other treatments have been suggested for dogs with AP, but little evidence exists to support their efficacy. Although bacterial translocation has been shown to occur in patients with pancreatitis, this complication tends to occur later in the disease process. Early, untargeted antibiotic therapy is not considered to be useful and should be avoided to prevent infections with resistant bacterial species.10 Similarly, the use of fresh frozen plasma or surgical intervention has not been shown to be effective in the treatment of AP in any species.11 Although corticosteroids have been shown to prevent neutrophilic extravascular traps, which is one of the hallmark findings in pancreatitis patients that develop respiratory failure, corticosteroids have many other potentially unwanted effects and thus their overall effect may be detrimental to the patient. There is minimal, weak evidence to support the routine use of corticosteroids for dogs with AP.12
PANOQUELL®-CA1
Fuzapladib sodium for injection (PANOQUELL®-CA1) is the first drug that has a reasonable expectation of efficacy for the management of the clinical signs of AP and is fully licensed for the treatment of clinical signs of AP in dogs in Japan and has recently received FDA conditional approval for use in dogs in the United States.13 Fuzapladib sodium is a leukocyte function-associated antigen-1 (LFA-1) activation inhibitor that prevents the extravasation of neutrophils from the capillary beds in the pancreas and extrapancreatic organs.14 Inhibition of inflammatory cell adhesion and migration into sites of tissue injury and inflammation helps to limit excessive inflammation. This can help prevent complications such as acute respiratory distress syndrome and multiorgan failure (Figure).
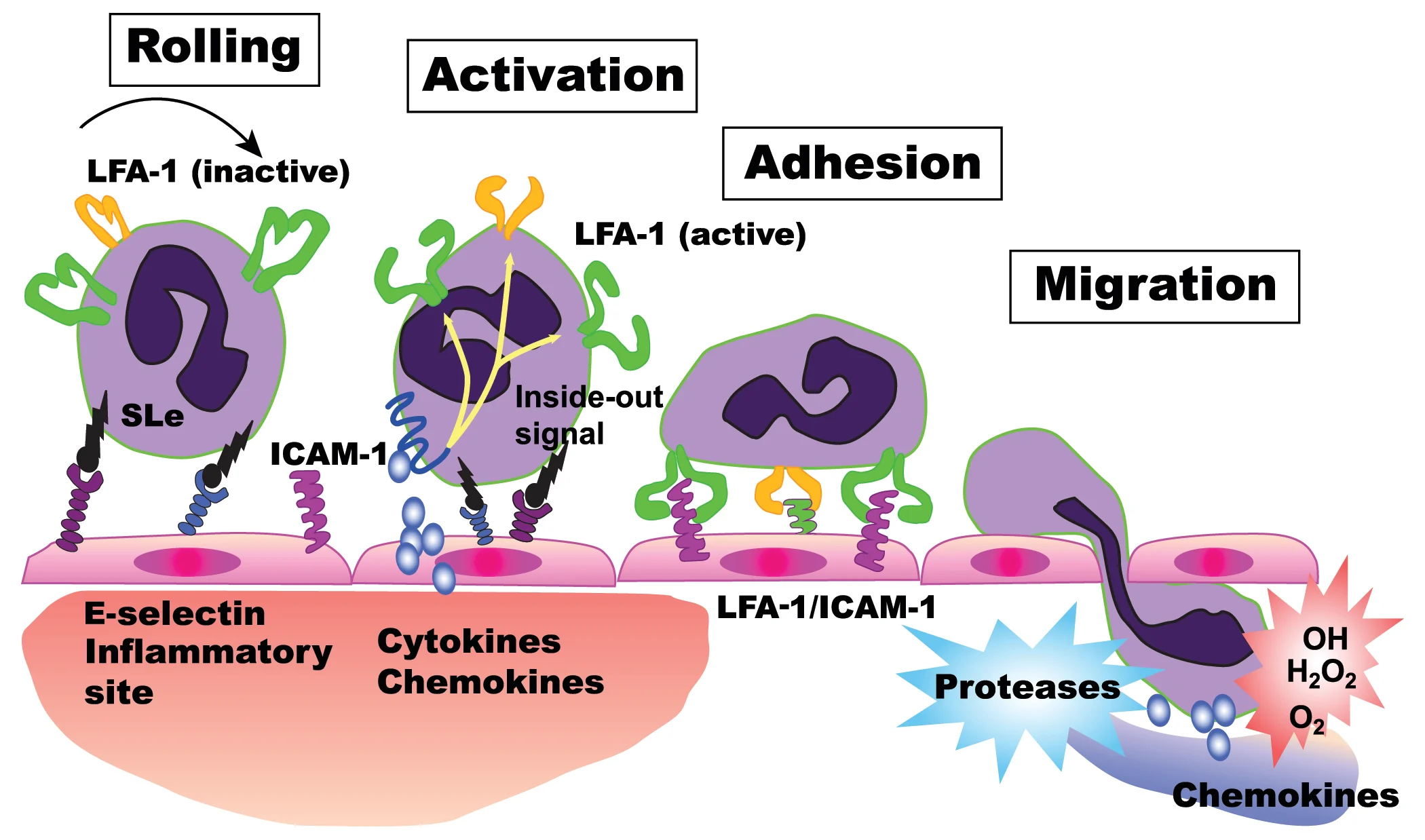
Mechanism of action of fuzapladib sodium. Fuzapladib sodium prevents the activation of LFA-1, which in turn prevents the attachment of neutrophils to ICAM-1 expressed on the surface of epithelial cells of the capillaries. This thereby prevents extravasation of neutrophils from the capillary beds into the surrounding tissue, reducing pancreatic inflammation.
A randomized, placebo-controlled, blinded pilot field effectiveness study in the United States showed that dogs with AP treated with fuzapladib sodium (0.4 mg/kg IV for 3 consecutive days) showed statistically greater clinical improvement as compared with dogs treated with a placebo.14
Although dogs that develop severe forms of AP would be the primary target for treatment with fuzapladib sodium, accurate prediction of these cases is not reliably possible.15,16 Several severity scoring systems have been developed for use in humans with AP, but none are sensitive or specific enough for early identification of patients that will move on to have severe disease. The risk for development of systemic complications or even death due to acute respiratory distress syndrome or systemic inflammatory response syndrome makes early treatment of AP (ie, before development of severe disease) in dogs with fuzapladib sodium a prudent approach.
Beyond the clinical impact, the financial impact of severe pancreatitis, which is often associated with dramatically longer hospital stays and associated costs, may be substantial for the pet owner. Further clinical studies are underway to confirm the clinical utility of fuzapladib sodium for dogs with AP.
PANOQUELL® is a registered trademark of Ishihara Sangyo Kaisha, Ltd.
