Elevated BUN After Chemotherapy
M. Katherine Tolbert, DVM, PhD, DACVIM (SAIM), Texas A&M University

Romeo, an 11-year-old, 11.6-lb (5.3-kg), neutered male crossbreed dog, was presented for a recheck evaluation for urinary bladder transitional cell carcinoma. He had previously undergone chemotherapy (mitoxantrone [5 mg/m2 IV q3wk]) for 4 months and definitive radiation therapy.
Romeo had a history of left cranial cruciate ligament rupture that had been conservatively and nonsurgically managed with deracoxib (1.4 mg/kg PO q24h) for the year prior to presentation for a bladder tumor; CBC and serum chemistry profile performed about a month between each visit were unremarkable (Table). His owners reported no problems, and he had a normal appetite with no history of vomiting or diarrhea.
Physical Examination
On physical examination, Romeo was bright, alert, and responsive. The only remarkable findings were left pelvic limb lameness and medial buttress of the left stifle. No abnormalities were noted on fundic examination, no pain was elicited on abdominal palpation, and rectal examination was unremarkable, with normal stool consistency and color.
SERUM CHEMISTRY RESULTS DURING DERACOXIB MONOTHERAPY & CHEMOTHERAPY & FOLLOWING RADIATION THERAPY
Diagnostic Findings
An increase in BUN and BUN:creatinine ratio levels was observed over ≈5 months prior to presentation for recheck evaluation. Because Romeo was able to tolerate deracoxib and had no outwardly detectable adverse effects from NSAID administration, his increased BUN was initially attributed to GI injury secondary to chemotherapy and radiation therapy, but no improvement was shown with omeprazole and sucralfate treatment following chemotherapy. Deracoxib was continued because of the severity of Romeo’s orthopedic disease and potential benefits of cyclooxygenase-2 inhibition in the treatment of bladder carcinomas. Although Romeo was clinically healthy, his BUN levels continued to increase following cessation of radiation therapy and during the weeks in which chemotherapy was not administered. He was treated with sucralfate slurry (500 mg PO q8h) and omeprazole (1 mg/kg PO q12h) for 2 weeks, and CBC and serum chemistry profile were evaluated at regular intervals; however, his BUN and BUN:creatinine levels continued to increase. A video capsule endoscopy (VCE) was performed to investigate the possibility of subclinical GI bleeding and revealed multiple hemorrhages in the distal small intestine (Figure; see Treatment at a Glance). Treatment with deracoxib was discontinued after VCE was performed.
VCE has been used in dogs with unexplained causes of microcytosis, iron-deficiency anemia, or hypoalbuminemia to determine whether the cause was related to GI bleeding.1 VCE should be reserved for dogs weighing >9.5 lb (4.3 kg) because the size of the capsule restricts its use in smaller dogs. VCE is not recommended in cats, even those weighing >9 lb (4 kg), because of gastric retention of the capsule. The sensitivity of VCE for detection of the source of GI bleeding in dogs is unknown but is likely less than that of traditional endoscopy; thus, VCE should only be used when more sensitive measures for detection of GI bleeding are unavailable.
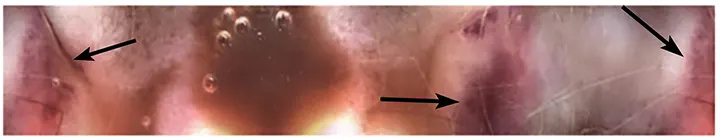
VCE confirming GI bleeding from multiple hemorrhagic areas (arrows) identified in the small intestinal mucosa
DIAGNOSIS:
SUBCLINICAL GI BLEEDING
Treatment & Long-Term Management
Omeprazole and sucralfate were continued; deracoxib was discontinued, and subsequent blood work revealed normalization of Romeo’s BUN and serum BUN:creatinine levels. Mitoxantrone therapy was continued for the next year, and his BUN and BUN:creatinine levels remained within normal limits.
TREATMENT AT A GLANCE
VCE is a noninvasive tool that can help confirm GI bleeding in dogs if traditional endoscopy is not available or if the dog is not a good anesthetic candidate.2
Fecal occult blood testing can increase the index of suspicion for GI bleeding in dogs but, because of associated low specificity, should not be used as a stand-alone test.
The treatment of choice for NSAID-induced upper GI bleeding is proton-pump inhibitor (PPI; eg, omeprazole, esomeprazole, pantoprazole) therapy at 1 mg/kg PO or IV q12h.3 This class of drugs is superior to H2-receptor antagonists (eg, famotidine, ranitidine) at equivalent doses in the treatment of GI ulceration in dogs.3
Prognosis & Outcome
At the 6-month follow-up after deracoxib was discontinued, Romeo had no evidence of GI bleeding and was still undergoing chemotherapy treatment for his bladder tumor. Because the GI bleeding was identified early and deracoxib was withdrawn, a good resolution is expected for Romeo’s NSAID-induced adverse effects.
Discussion
Adverse GI effects (eg, vomiting, diarrhea) are relatively common with NSAID administration in dogs, but less common adverse effects (eg, GI bleeding) may not always be detectable by the owner. Patients with multiple risk factors (eg, older dogs, dogs with comorbidities) are likely at increased risk. Early detection and treatment and, when possible, discontinuation of the NSAID can help ensure a good prognosis in dogs with NSAID-induced GI adverse effects (see Take-Home Messages).
TAKE-HOME MESSAGES
The most common NSAID-induced adverse effects in dogs are GI in origin.4
Outwardly detectable adverse effects are not always present in dogs with NSAID-induced GI bleeding. Increased BUN and BUN:creatinine levels can be early indicators of subclinical GI bleeding and can occur in the absence of anemia.5
Patients with multiple risk factors (eg, older dogs, dogs receiving ulcerogenic or erosive drugs, dogs with GI comorbidities such as inflammatory bowel disease) are at the highest risk for NSAID-induced GI bleeding.6 Dogs without other risk factors for GI bleeding that receive NSAIDs at appropriate doses, especially when used for short periods of time, rarely experience adverse effects.4 In the absence of additional risk factors for NSAID-induced GI injury, NSAIDs should be used alone. In the absence of other risk factors for GI bleeding, prophylactic PPI therapy with NSAID administration is not recommended because, although PPIs can help control GI bleeding in the stomach and proximal duodenum, the combination of NSAIDs with PPIs may worsen distal intestinal bleeding. This is thought to be secondary to alteration of the intestinal microbiota, increased intestinal permeability, and increased intestinal inflammation.3,7
Discontinuation of NSAIDs and treatment with PPIs are recommended when possible for dogs presented with NSAID-induced clinical GI bleeding.
PPI = proton-pump inhibitor, VCE = video capsule endoscopy