
Successful identification of an arrhythmia requires understanding the cardiac conduction system.
A normal working rhythm includes the following:
Heart rate in a normal or expected range for the breed, species, and clinical situation (eg, sinus arrhythmia and/or wandering pacemaker are considered normal in a relaxed dog but are not normal in a cat being examined in a clinical setting; Figures 1-3)
Rhythm in which there is a P wave for each QRS complex, a QRS complex for each P wave, and (most importantly) a relationship between the P waves and QRS complexes
Constant atrioventricular (AV) interval
QRS complex that appears upright and narrow in leads II, III, and aV
P wave that appears upright in leads I, II, III, and aVF.

ECG showing a normal sinus rhythm

ECG showing a sinus arrhythmia with a regularly irregular rhythm, in which the heart rate increases and decreases in a pattern. This is considered normal in relaxed dogs.

ECG and illustrations showing a wandering pacemaker. Tall P waves with fast heart rates and high sympathetic tone and short P waves with slow heart rates and high vagal tone can be seen. This is considered normal in relaxed dogs.
Related article: Interpreting ECGs with Confidence
Clinical Response to an Abnormal Rhythm
In patients with an abnormal ECG, whether the arrhythmia is hemodynamically important (eg, affecting cardiac output, in a breed with known risk for sudden death) should initially be determined. An arrhythmia that has not resulted in cardiac enlargement is likely not hemodynamically affecting the patient but may indicate presence of underlying heart disease, warranting echocardiographic evaluation.
Bundle Branch Block
The ECG hallmark of a bundle branch block is a markedly widened QRS complex (dogs, ≥80 ms in duration on ECG; cats, >40 ms in duration on ECG). Although these blocks have a unique pattern of conduction, they are supraventricular in origin (ie, sinus rhythm in which the impulse has been initiated from the SA node and conduction is through the AV node but with a different pattern) and should not be misinterpreted as ventricular tachycardia (VT). Identification can be accomplished by noting a P wave associated with the QRS complex and a constant PR interval (Figure 4).

Illustration and representative ECGs of a right bundle branch block (RBBB) and a left bundle branch block (LBBB). QRS complexes are wide in both blocks, but a P wave is present for each QRS complex, a QRS complex is present for each P wave, and the PR interval is constant. With an RBBB, impulse formation travels down the left side normally, but the right ventricle is delayed in depolarization because it has to use slow muscle cell to muscle cell conduction; depolarization and repolarization of the right ventricle therefore take longer than normal (≥80 ms measured on ECG). Impulse formation is complete after the right ventricle is depolarized by the impulse moving from the apex toward the base of the right side of the heart, creating a right axis shift and an ECG with deep S waves in leads II, III, and aVF but a constant PR interval and normal heart rate. With an LBBB, there is no axis shift because ventricular depolarization typically occurs toward the left apex. A markedly prolonged QRS complex, normal and constant PR interval, normal mean electrical axis, and normal heart rate are seen instead.
Atrioventricular Nodal Block
Evaluation of an arrhythmia with AV nodal disease includes determination of whether the arrhythmia is hemodynamically important (ie, rhythm is slow enough to cause clinical signs, including exercise intolerance, syncope, and signs of congestive heart failure) and whether there is autonomic influence on the arrhythmia that can be accomplished with administration of a high-end dose of atropine (0.04 mg/kg SC or IM). Expected response for a normal sinus or AV node with excessive vagal influence is complete resolution of the AV block and a heart rate >160 to 180 bpm (Figure 5).
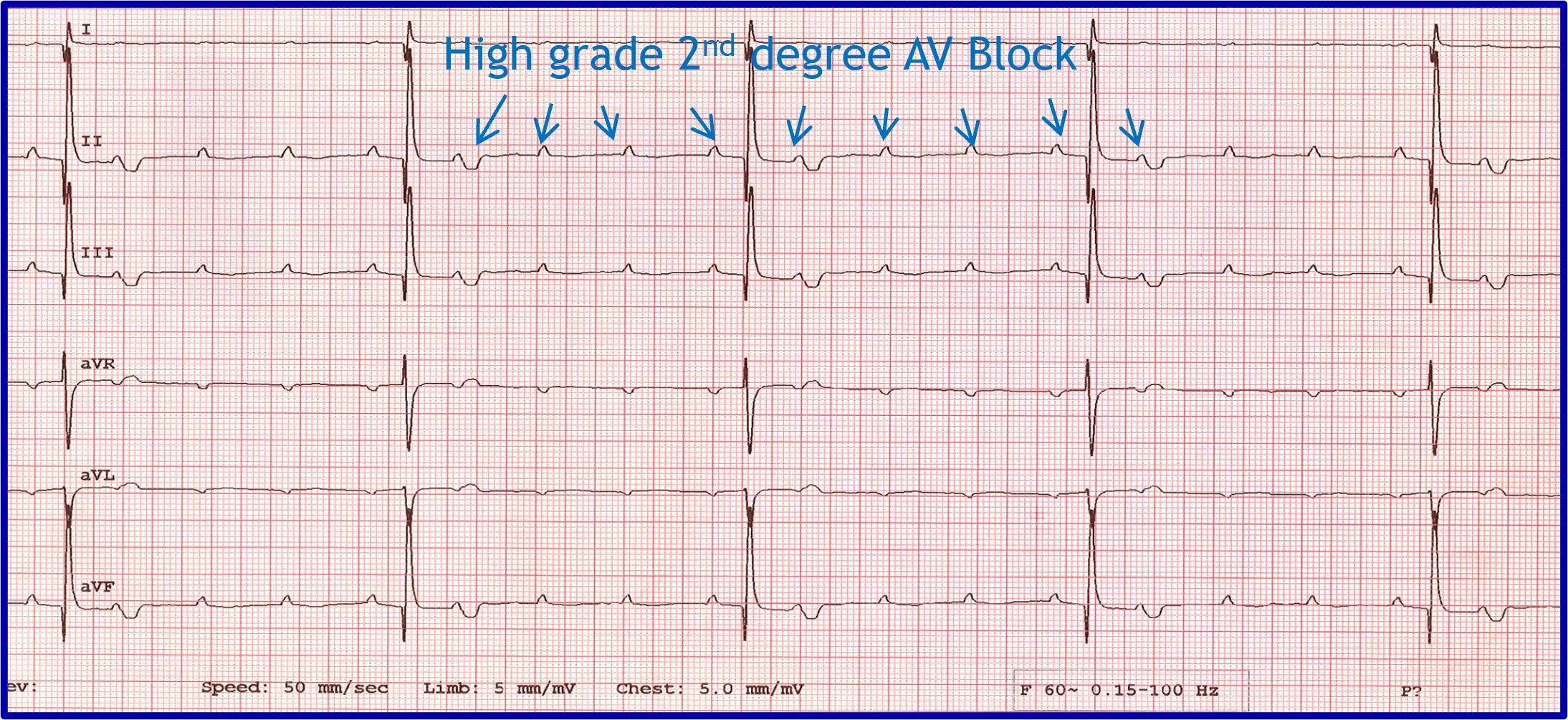
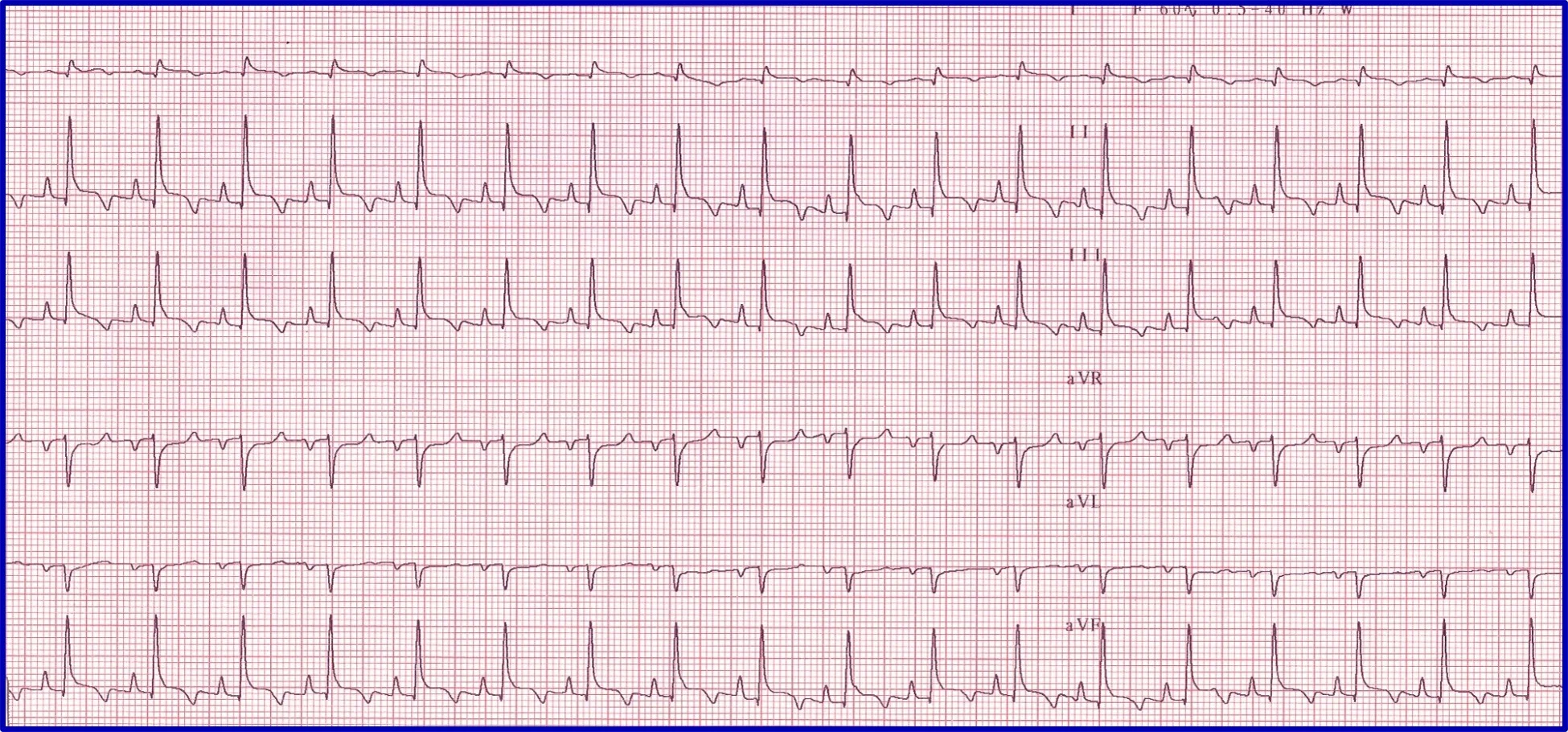
(A) ECG in a patient with high-grade second-degree AV block in which no 2 consecutive P waves are conducted to create ventricular depolarization. Each QRS complex has a P wave with a constant PR interval, but many P waves do not have an accompanying QRS complex. (B) Thirty minutes after administration of atropine (0.04 mg/kg IM), ECG showed complete resolution of the AV block, 1:1 conduction of every P wave, and heart rate >180 bpm, indicating the SA and AV nodes are fully functional and the prior delay in conduction was vagally mediated.
Premature Beats
Premature beats occur abnormally fast compared with the previous beat and other prior beats and can be individual beats, couplets, triplets, or runs of tachycardia. Evaluation should include assessment of where the premature beats originated and how they traveled within the heart.
Premature beats originating at or above the AV junction can still be rapidly conducted to the ventricular myocardium via specialized conduction tissue and therefore appear nearly identical to the regular rhythm. Premature beats originating below the AV junction (ie, below the bundle of His) cannot use the specialized conduction system and must therefore depolarize the ventricles via muscle cell to muscle cell conduction. This is a relatively slow process that produces a wide, bizarre QRS complex that can be predominantly positive or negative depending on where and in which ventricle the impulse starts and what direction it travels from to allow for complete ventricular depolarization. There will not be a related P wave because the impulse forms and depolarizes independently from atrial depolarization. Because depolarization is abnormal, repolarization is also abnormal and is represented by a large, bizarre T wave on ECG (Figure 6).
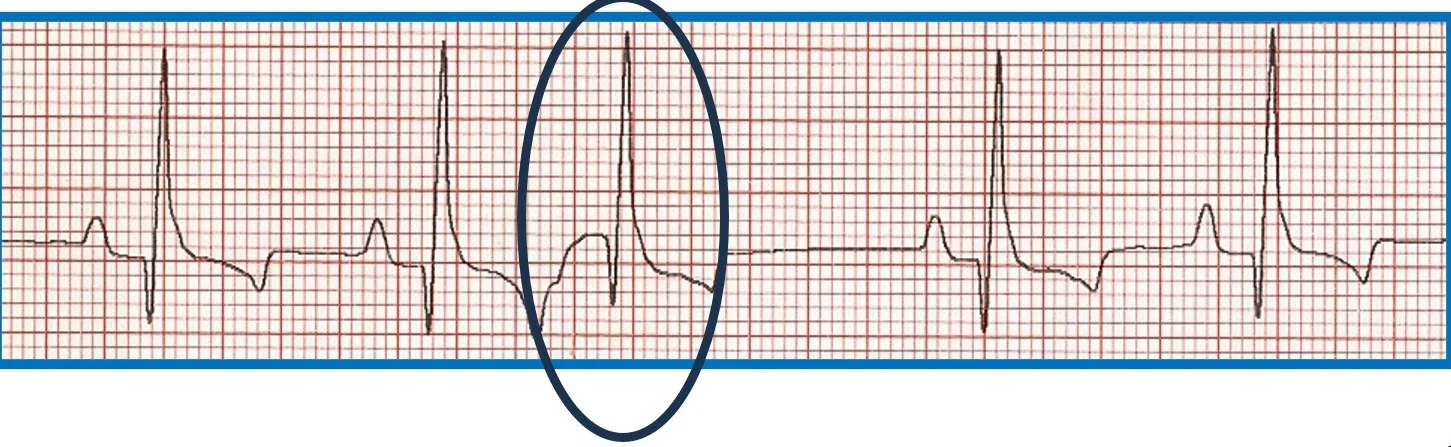
FIGURE 6 Effect of ectopic foci origination location on ECG appearance. (A) ECG showing a normal QRS complex but indiscernible P wave (circle) characteristic of premature beats with supraventricular origin; the circled premature beat is almost identical to the beats with an associated P wave. (B) Illustration demonstrating where ectopic foci result in supraventricular (above dotted line) and ventricular (below dotted line) ECG patterns. (C) ECG showing no P wave; a wide, bizarre QRS complex; and a large, bizarre T wave (circles) characteristic of premature beats with a ventricular origin.
Accelerated Idioventricular Rhythm
Runs of ventricular ectopy are not always dangerous. Many systemic diseases can cause accelerated idioventricular rhythm (AIVR; ie, slow VT; Figure 7). Rhythm rate is key for distinguishing AIVR from dangerous VT. AIVR rate is typically <180 to 200 bpm, and VT is typically >250 bpm. Couplets, triplets, or runs with a rate between 200 and 250 bpm indicate a possible need for therapy in patients with no concurrent systemic or underlying cardiac disease that could account for the rhythm disturbance.

ECG showing an AIVR rhythm in a Lhasa apso with immune-mediated hemolytic anemia
Sick Sinus Syndrome/Sinus Node Dysfunction
Sick sinus syndrome (SSS)/sinus node dysfunction (SND) is a conduction disease of the SA node believed to be degenerative. Other regions of the conduction system may also be diseased. Escape rhythms do not always, therefore, appropriately rescue the rhythm. ECG features of SSS/SND include sinus arrest (ie, cessation of SA node activity/no atrial activity or P waves identified and absence or delay of expected escape/rescue rhythms from other automatic tissues in the heart [eg, bundle of His, Purkinje fibers, ventricular tissue]; Figure 8). For example, the intrinsic rate of the AV node is 40 to 60 bpm. If the SA node pauses >1.2 seconds, the AV junction should depolarize and produce a junctional beat. Likewise, if the SA node (intrinsic rate, 30 bpm) pauses for >2 seconds, the Purkinje fibers should fire and rescue the rhythm; however, this often does not occur or is inappropriately delayed in patients with SSS/SND, and sinus arrest/asystole can last for several seconds. Junctional rhythms may become the dominant rhythm. Administration of a high-end dose of atropine (0.04 mg/kg SC or IM) may result in an increase in heart rate. Lack of an appropriate response (ie, AV block resolves, sinus rate increases to >180 bpm, every P wave is associated with a QRS complex, PR intervals are constant) is diagnostic for SSS/SND; however, a partial or even complete response does not necessarily rule out SSS/SND. SSS/SND is highly influenced by autonomic tone; therefore, a partial or full response to atropine in a breed predisposed to SSS/SND (eg, West Highland white terriers, schnauzers, dachshunds, cocker spaniels) with clinical signs and ECG or Holter characteristics of SSS/SND does not exclude this diagnosis.
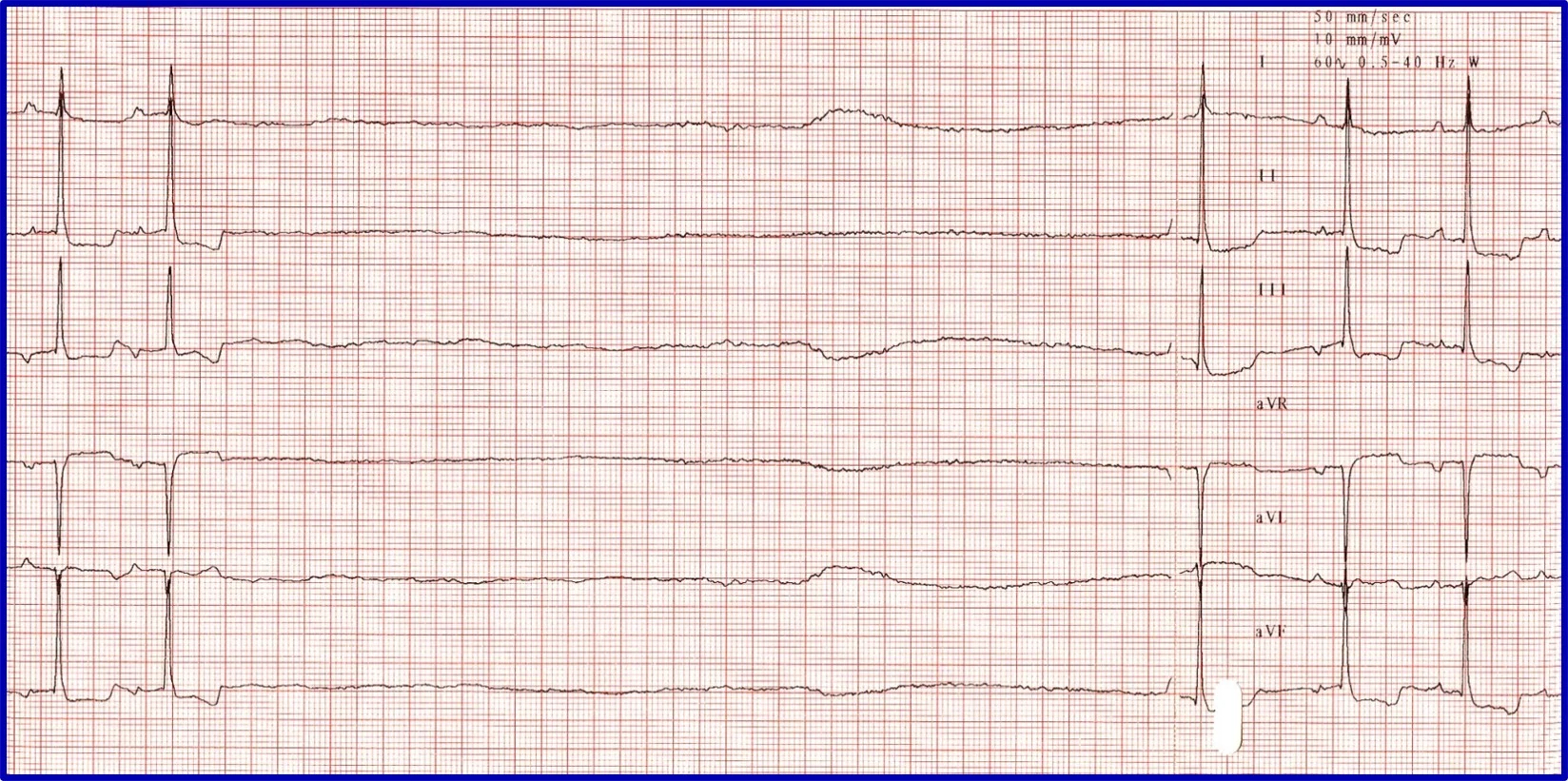
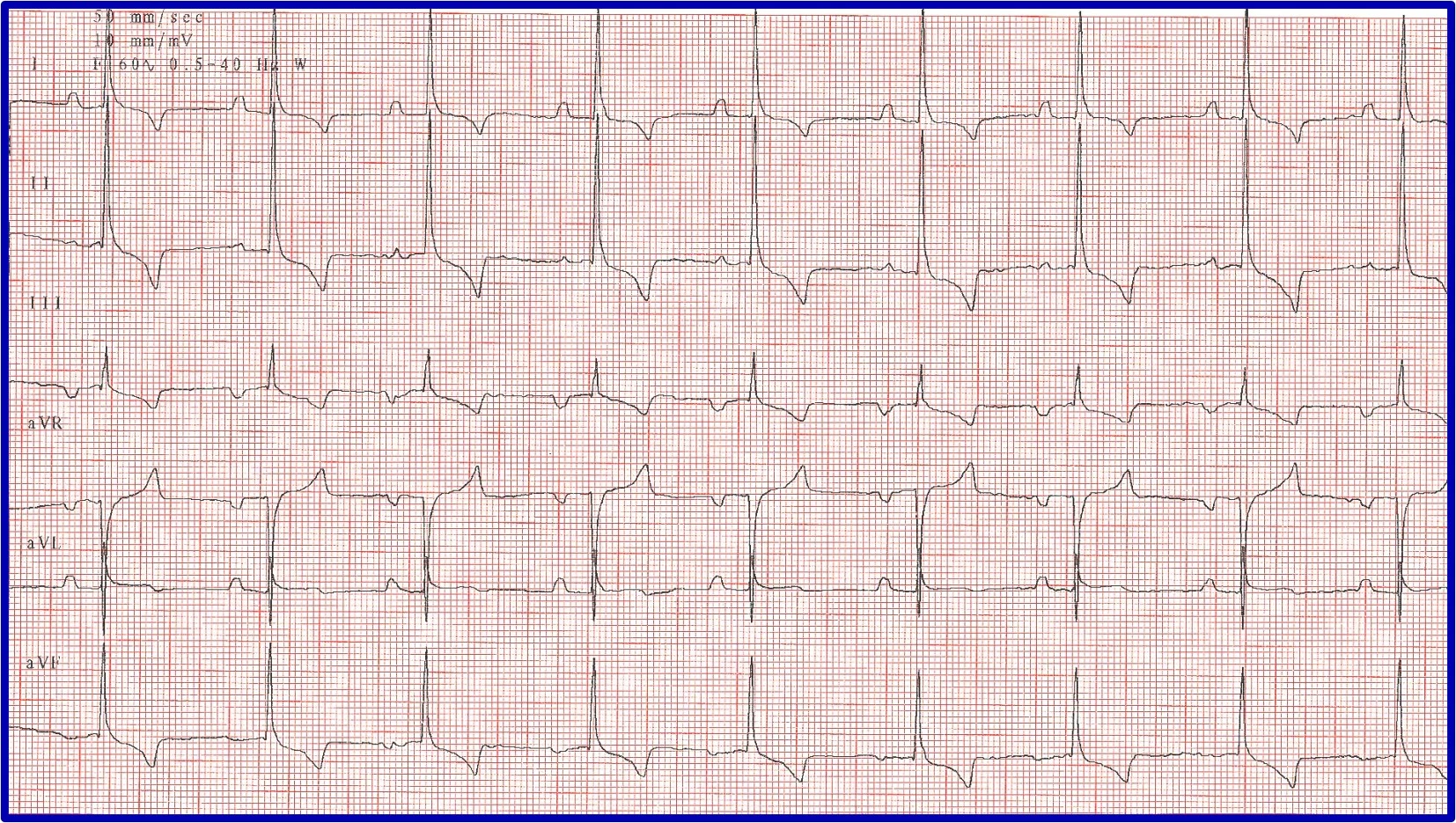
(A) ECG in a patient with SSS/SND and a delay of escape focus to rescue the rhythm. (B) Thirty minutes after administration of atropine (0.04 mg/kg IM), ECG showed resolution of SSS/SND, but the SA node was only able to speed up to 140 bpm, indicating that although the dysfunction was heavily influenced by autonomic tone, there was an incomplete response to obliterating vagal tone. The SA node was not able to operate at full function.
Atrial Fibrillation
ECG features of atrial fibrillation (AF) include an irregularly irregular rhythm (variable RR intervals) with no identifiable pattern. P waves cannot be reliably identified, and heart rate is usually >200 bpm. QRS complexes typically appear to be supraventricular (ie, narrow and upright in lead II); however, some deep-chested breeds (eg, Doberman pinschers, Irish wolfhounds) develop wider QRS complexes with left ventricular enlargement instead of a tall QRS. The baseline may have irregular undulations (ie, fibrillation waves), but this is not a requirement for diagnosis. Fibrillation waves are not always present or identifiable, especially when the heart rate is fast. Aberrantly conducted beats may cause variation in the height and morphology of QRS complexes (Figure 9).

ECG in a patient with AF and aberrant ventricular conduction. A fusion beat (ie, simultaneous ventricular premature complex and normal sinus beat that results in an ECG trace that is a sum of the 2 vectors of depolarization) can be seen (circle). With AF, the atria fibrillate at 500 to 600 bpm; the AV node cannot discern whether it should conduct impulses and thus attempts to conduct all impulses but is unable to do so because it cannot depolarize and repolarize at that rate. Beats that do not appear supraventricular in origin were conducted when only parts of the conduction system were repolarized and ready to conduct an impulse from the AV node to the ventricular myocardium; these impulses take an abnormal pathway to the ventricle, resulting in the appearance of a wide, bizarre ventricular beat (arrows). For example, if the right bundle branch is repolarized and ready to conduct while the left bundle is still in a refractory period, an ECG complex that resembles an LBBB occurs. A different conduction pattern is possible with almost every beat, as the right and left bundles can be at different phases of refractoriness when each impulse is presented. This type of aberrant conduction is common with AF and important to recognize because it is not a dangerous ventricular rhythm and should not be treated with lidocaine or other ventricular antiarrhythmic therapy.
Irregularity can be difficult to identify when AF rate is >250 bpm. Multiple ECGs or ECGs with multiple leads (eg, 6- or 12-lead ECG) are needed. ECG recorded at a faster speed (eg, 50 mm/second) can also be helpful. A rapid, irregular supraventricular tachycardia without identifiable P waves should be considered AF until proven otherwise. If an alternative diagnosis is possible, AF should be ruled out first, as it is the most likely possibility.
Ventricular Arrhythmia
Although identifying ventricular arrhythmias (ie, malignant ventricular premature complexes, VT) is not difficult, deciding whether and when to treat a ventricular ectopic rhythm can be challenging.
It is important to discern whether the arrhythmia is causing hemodynamic compromise by evaluating peripheral pulse quality, mucous membrane color, patient behavior, and arterial blood pressure. Sustained VT at a high rate is more likely to cause hemodynamic compromise than VT at a slower rate or AIVR (Figure 10). Determining whether the arrhythmia is likely to degenerate into ventricular fibrillation, which causes sudden death, is also important. Increased speed of VT or ventricular ectopic beats increase the likelihood that a beat will fall within the vulnerable period and induce ventricular fibrillation (ie, R on T phenomenon).
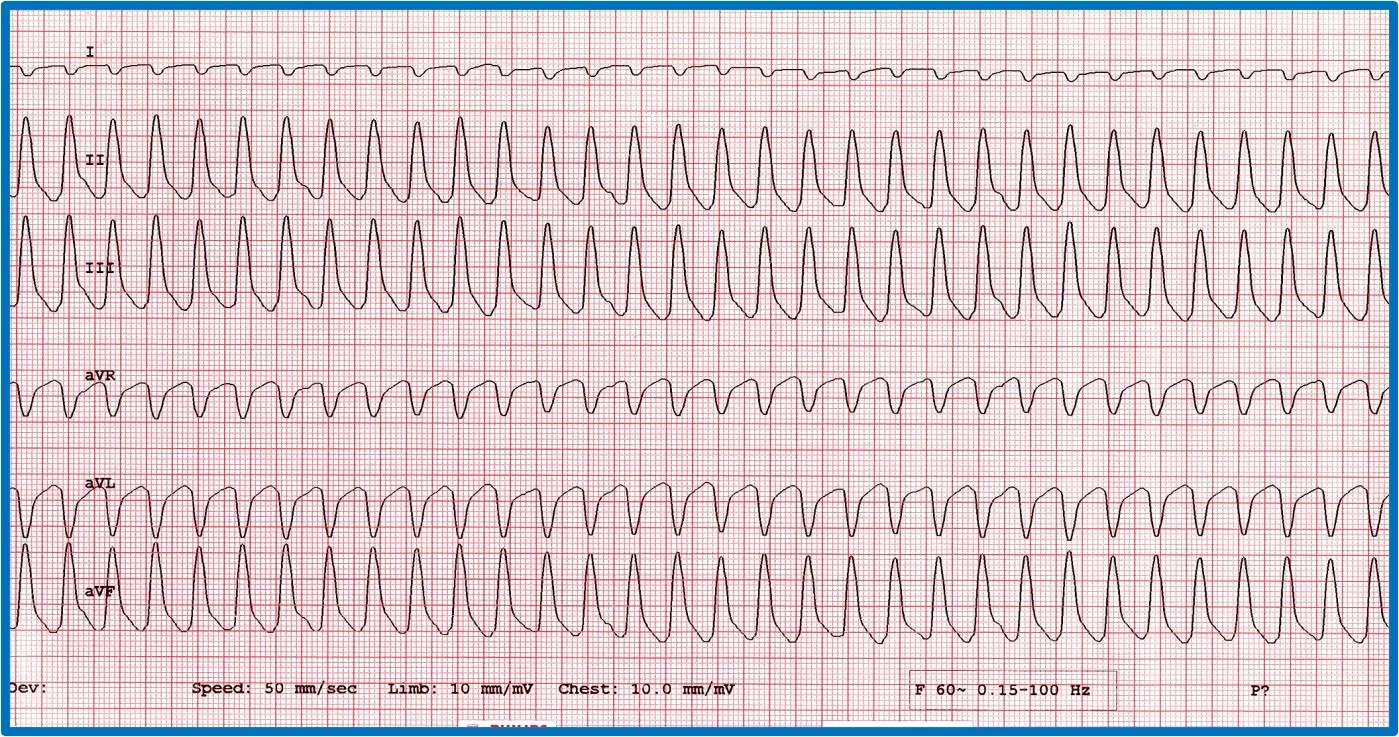
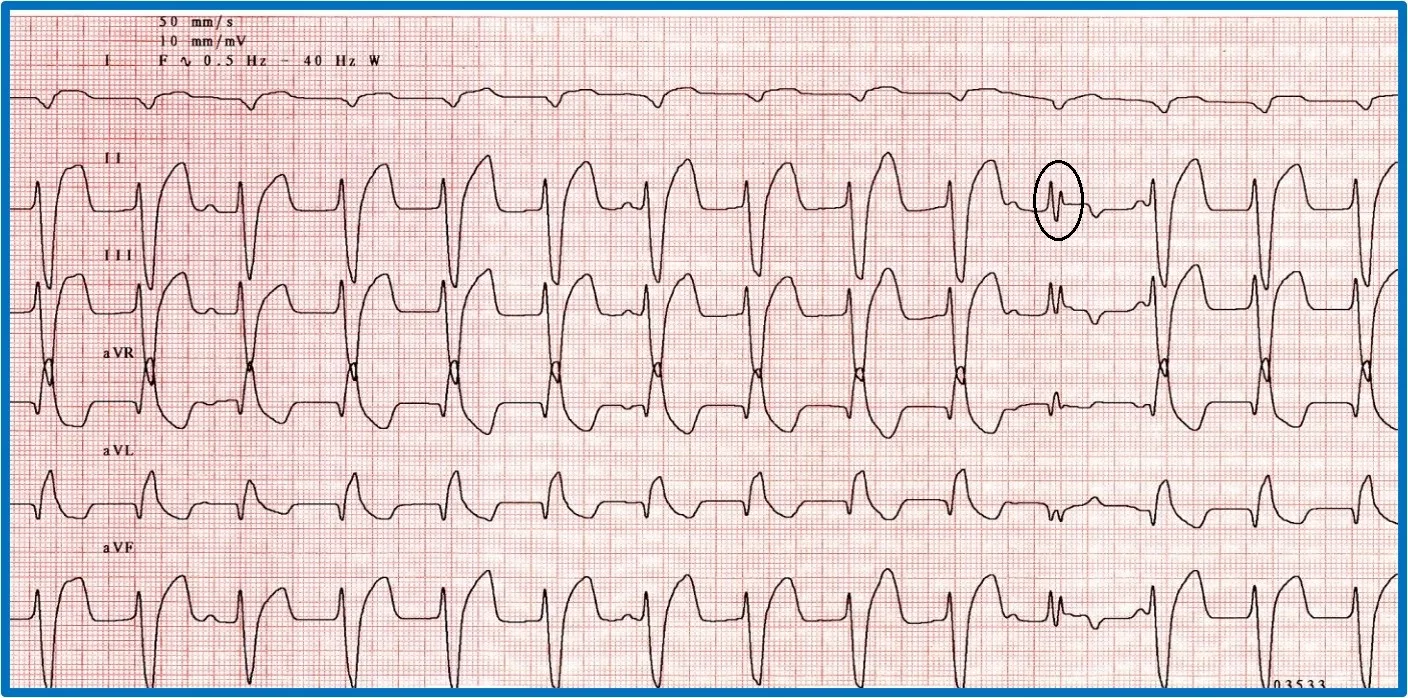
Two versions of ventricular runs of ectopy. (A) ECG showing a run of sustained true VT with a rate of 320 bpm in a boxer. (B) ECG showing an AIVR rhythm (ie, slow VT) in a crossbreed dog hit by a car and experiencing traumatic myocarditis. The rate is 180 bpm and not hemodynamically compromising the patient. A sinus beat occuring at the same time as a ventricular beat, causing a fusion beat (circle), can be seen.
Polymorphic VT, ventricular premature complexes that occur in couplets at a fast coupling rate, and ventricular ectopy are thought to be more dangerous than monomorphic VT or ventricular ectopy. Certain patient groups (eg, Doberman pinschers with dilated cardiomyopathy, boxers with arrhythmogenic right ventricular cardiomyopathy, patients with severe subaortic stenosis, German shepherd dogs with inherited ventricular arrhythmias, cats with hypertrophic cardiomyopathy) may have an increased risk for sudden death associated with VT and ventricular ectopy; treatment of ventricular arrhythmias in these patients is therefore typically recommended.
Listen to the Podcast
Dr. Estrada joined Clinician's Brief: The Podcast to share more about how to address these arrhythmias in general practice.