
Walt, a 2-year-old, 128-lb (58-kg) intact male Great Dane, was presented for routine neuter. The owner reported no medications had been administered except an oral monthly heartworm preventive.
Physical Examination
Physical examination was unremarkable. Temperature was 101.9°F (38.9°C), and heart rate was 120 bpm. The patient was panting. Preanesthetic blood work (packed-cell volume, serum chemistry profile) was within normal limits.
Sedation
Walt was resistant to physical restraint, and excessive restraint would have been needed for IV catheterization. IM premedication was thus elected, and a combination of 0.25 mg dexmedetomidine (4.3 micrograms/kg) and 6 mg hydromorphone (0.1 mg/kg) was administered in the lumbar epaxial muscles. Profound sedation was noted after 20 minutes, and an IV catheter was placed in the left cephalic vein.
General anesthesia was induced with 100 mg propofol (1.7 mg/kg IV). A 14-mm (internal diameter) endotracheal tube was placed, and the cuff was inflated to effect until positive-pressure ventilation could be held at 20 centimeters of water. Isoflurane (initial setting on vaporizer, 1.5%) was delivered in 100% oxygen (1-2 L/min) via a rebreathing system, and positive-pressure ventilation was initiated to maintain end-tidal carbon dioxide (ETCO2) between 40 and 45 mm Hg.
Read about the Top 5 Short Procedure Sedation Scenarios, including scenarios for fractious or aggressive dogs or cats.
Walt was immediately positioned in dorsal recumbency. A pulse oximetry probe was placed on the tongue, and blood pressure was measured using an oscillometric monitor with a #5 cuff over the dorsal pedal artery. Initial heart rate was 27 bpm, oxygen saturation was 100%, and mean arterial pressure (MAP) was 115 mm Hg (Figure 1).
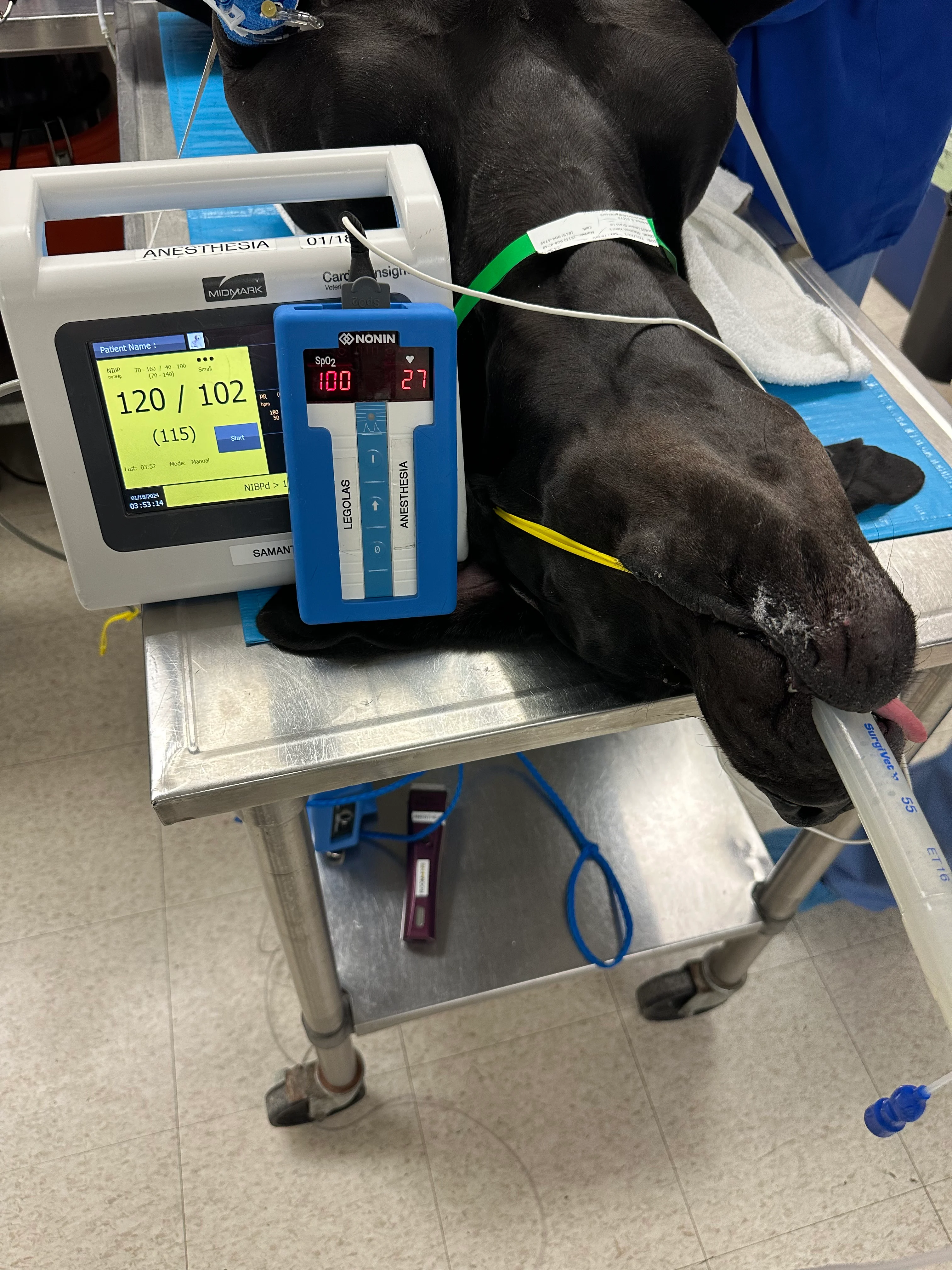
Patient under anesthesia prior to routine neuter. Diastolic arterial pressure is elevated (102 mm Hg), which may indicate vasoconstriction; however, noninvasive (oscillometric) blood pressure monitors may be prone to artifact in cases of bradycardia and/or vasoconstriction, which are common with dexmedetomidine administration.
Palpation revealed a strong but inconsistent femoral pulse with an overall heart rate of 40 bpm (range, 27-45 bpm due to occasionally absent pulses). Auscultation with simultaneous peripheral pulse palpation revealed multiple pulse deficits. ECG showed an underlying sinus rhythm and occasional second-degree atrioventricular (AV) block (Figure 2).

ECG showing an underlying sinus rhythm and occasional second-degree AV block (asterisk)
Diagnosis: Bradycardia With Second-Degree Atrioventricular Block
Diagnosis
Bradycardia with intermittent second-degree AV block was present with concurrent normotension (bordering on hypertension), likely due to the cardiovascular effects of dexmedetomidine, which can cause a biphasic blood pressure response.1 Initial peripheral vasoconstriction led to an increase in systemic vascular resistance (SVR) and thus an increase in MAP. Increased SVR and increased vagal tone resulted in a decreased heart rate, which is a common effect of dexmedetomidine.
Treatment
The decision to treat bradycardia in a patient under anesthesia is often not straightforward. Bradycardia may be borderline, and treatment may cause risk for iatrogenic hypertension.
Although initial pulse palpation and ECG revealed an overall heart rate of 40 bpm (which alone does not necessarily warrant treatment), ongoing monitoring revealed multiple occurrences of second-degree AV block with overall heart rate <30 bpm. The bradycardia was accompanied by a significant bradyarrhythmia. Glycopyrrolate (0.005 mg/kg IV) was administered.
Treatment at a Glance
Treatment for bradycardia in an anesthetized patient is typically advised when bradycardia is severe (<40 bpm), significant bradyarrhythmias are present, or bradycardia is accompanied by hypotension.
Anticholinergic agents (eg, atropine, glycopyrrolate) can be administered; however, underlying mechanisms of bradycardia should be considered.
Onset of glycopyrrolate may be delayed, even after IV administration; effects may not occur for 5 to 7 minutes. Atropine is indicated if urgent treatment is needed.
Outcome
After 7 minutes, heart rate increased to 90 bpm, sinus rhythm was noted on ECG, and blood pressure was 127 mm Hg. ETCO2 increased from 40 mm Hg to 45 mm Hg. The remainder of the procedure and recovery were uneventful.
Discussion
Bradycardia is defined by a heart rate <50 bpm in awake dogs and is relatively common in anesthetized dogs.2 Further diagnostic testing is often required to elucidate the underlying mechanism. Differential diagnoses may include hypothermia, drug-induced bradycardia (eg, alpha-2 adrenergic receptor agonists, opioids), electrolyte abnormalities (eg, hyperkalemia), increased vagal tone from surgical stimuli (eg, oculocardiac reflex), and systemic disease (eg, GI disease).
ECG allows rapid assessment of the presence of P waves associated with QRS complexes (ie, sinus bradycardia), P waves intermittently not associated with QRS complexes (ie, second-degree AV block), or absent P waves (ie, sinus arrest) or P waves not associated with QRS complexes (ie, third-degree AV block) and can help determine whether the underlying rhythm is sinus and whether a bradyarrhythmia (eg, AV block) is present. Palpation of a pulse should simultaneously be performed to determine the presence of pulse deficits. Complete discussion of dysrhythmias is available in the literature.2
Treatment decisions can be guided by equations that relate cardiac output, heart rate, stroke volume, SVR, and MAP (see Equation). For example, bradycardia may be tolerated or left untreated when there is concurrent normotension or hypertension, but hypotension may indicate bradycardia is significant and contributing to decreased cardiac output.
Cardiac output = heart rate × stroke volume
Mean arterial pressure = cardiac output × systemic vascular resistance
In cases of bradycardia with accompanying hypotension, a patient under anesthesia may require initial treatment. It is important, however, to note that although blood pressure may be an indirect indicator of cardiac output, MAP may be altered with concurrent changes in vascular tone (SVR). Hyper-, hypo-, or normotension may therefore be present with normal cardiac output, depending on the vascular tone. For example, hypotension is often present in patients given inhalant anesthesia because volatile anesthetics (eg, isoflurane) can decrease SVR.3
Alpha-2 adrenergic receptor agonists exert effects via the alpha-2 adrenergic receptors. Agonist action on the alpha-2A and alpha-2B receptor subtypes in the CNS result in sedation.4 Vasoconstriction mediated by the presence of alpha-2B receptors in vascular smooth muscle and other effects (eg, analgesia, decreased sympathetic outflow) are possible. Sedative agents, including dexmedetomidine and xylazine, have variable specificity for alpha-2 versus alpha-1 receptors, with activation of alpha-1 receptors resulting in vasoconstriction. Vasoconstrictive effects often result in increased SVR and MAP; subsequent baroreceptor-mediated bradycardia results in a physiologic mechanism to prevent or minimize significant hypertension. Visible manifestations of vasoconstriction (eg, dusky or bluish mucous membranes), difficulty in obtaining pulse oximetry readings, and difficulty with venipuncture or venous catheterization may be present, particularly following administration of higher doses of alpha-2 adrenergic receptor agonists. Concurrently, decreased sympathetic outflow can result in a dominance of parasympathetic (vagal) tone, decreasing heart rate and potentially resulting in bradyarrhythmias.1
Hemodynamic changes typically include decreased heart rate and increased blood pressure. In one study of dogs undergoing ovariohysterectomy, results showed an 80% incidence of bradycardia following dexmedetomidine administration, with increased likelihood of bradycardia development following dexmedetomidine administration compared with acepromazine.5 A study of healthy dogs wearing Holter monitors revealed a variable occurrence of second-degree AV block following dexmedetomidine administration, with some dogs demonstrating a moderate to frequent occurrence (minimum heart rate, 29 bpm).6
When considering treatment options, assessing the overall clinical picture (including ECG and blood pressure measurements) and determining whether bradycardia is severe enough to warrant treatment are important. Treatment may consist of an alpha-2 adrenergic receptor antagonist that can reverse the effects of dexmedetomidine. IM atipamezole can reverse sedation in patients given alpha-2 adrenergic agents; however, administration to patients under anesthesia may not be of hemodynamic use. One study assessed the effects of IM atipamezole in anesthetized cats following IV dexmedetomidine; treatment did not improve heart rate, cardiac output, or SVR.7 Dexmedetomidine antagonism can also result in loss of sedation and analgesia that contribute to a more balanced anesthetic and perioperative period.
Treatment of bradycardia may be accomplished with anticholinergics. Glycopyrrolate or atropine may be indicated in cases of significant bradycardia. A physiologic cause of bradycardia is a baroreceptor-mediated response to hypertension. Ablation of this response by exogenous agents (eg, anticholinergics) can result in unopposed hypertension. In a study in which atropine was pre-emptively administered prior to medetomidine (racemic mixture of levomedetomidine and dexmedetomidine), incidence of bradycardia and second-degree AV block was decreased, but blood pressure was significantly increased (peak systolic blood pressure, up to 260 mm Hg).8 A similar study found that coadministration of atropine and dexmedetomidine in dogs prevented bradycardia but resulted in significant hypertension with no improvement in cardiac output. In addition, multiple dogs developed ventricular arrhythmias following the combination of atropine and dexmedetomidine.9 When choosing anticholinergics, the hemodynamic status of the patient should be considered. If severe bradycardia or evidence of decreased blood pressure and/or cardiac output is present, treatment with anticholinergics may be warranted. Indiscriminate use should be avoided because the presence of underlying pathology is important when using alpha-2 adrenergic agonists and anticholinergics. Patients with underlying cardiac disease that cannot tolerate increases in myocardial oxygen demand with decreased cardiac output and increased afterload may decompensate with the combination of these agents.
Lidocaine has been proposed as a treatment for dexmedetomidine-induced bradycardia in dogs.10 An IV bolus and CRI of lidocaine following dexmedetomidine sedation improved heart rate and cardiac index in research dogs.10 Although further research may be required to determine the effect in patients and various clinical scenarios, lidocaine may be considered for treatment of dexmedetomidine-induced bradycardia.
Nonspecific indicators of poor perfusion (eg, cold extremities, decreased ETCO2, abnormal mucous membrane color) may indicate decreased cardiac output. Treatment with anticholinergics or lidocaine should be considered in cases of moderate to severe bradycardia (eg, <40 bpm), hypotension (MAP <60 mm Hg), or significant bradyarrhythmias.
Take-Home Messages
Dexmedetomidine may cause bradyarrhythmias, including sinus bradycardia and second-degree AV block.
Bradycardia in anesthetized patients may be tolerated or treated, depending on multiple factors, including blood pressure, indicators of decreased cardiac output, and severity of bradycardia.
Dexmedetomidine-induced bradycardia may be treated using anticholinergics or lidocaine. Administration of drug antagonists (eg, atipamezole) may be considered but may not be of hemodynamic benefit in anesthetized patients.
Listen to the Podcast
Dr. Schroeder discusses the physiological mechanisms underlying bradycardia and dexmedetomidine's biphasic blood pressure effects.