Management of Myxomatous Mitral Valve Disease
Sponsored by Ceva Animal Health, LLC
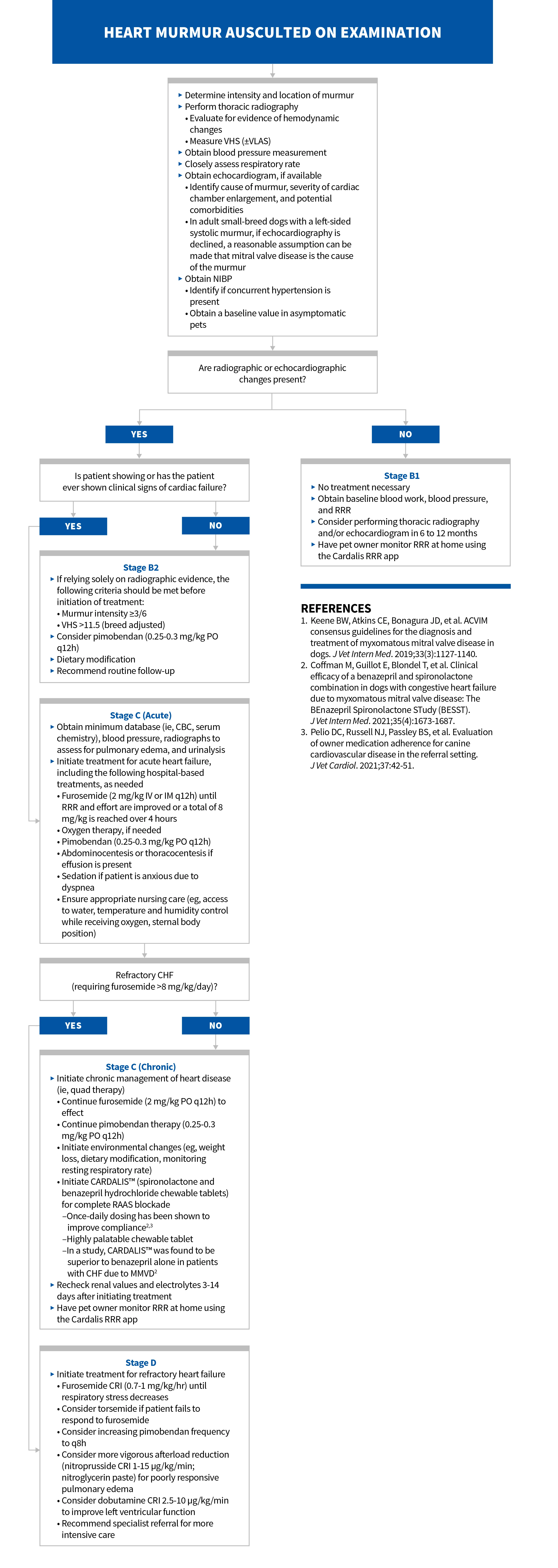
Myxomatous mitral valve disease is to blame for ≈75% of heart disease cases in our canine patients. The next time you have an affected patient, reference this flowchart for streamlined staging and management. Download it here.
CARDALIS™ is indicated with concurrent therapy (eg, furosemide) for the management of clinical signs of mild, moderate, or severe congestive heart failure in dogs due to atrioventricular valvular insufficiency (AVVI).
CARDALIS™ trademark is the property of Ceva Santè Animale S.A.
IMPORTANT SAFETY INFORMATION Do not administer in conjunction with non-steroidal anti-inflammatory drugs (NSAIDs) in dogs with renal insufficiency. Do not use in dogs with hypoadrenocorticism (Addison’s disease), hyperkalemia or hyponatremia. Do not use in dogs with known hypersensitivity to ACE inhibitors or spironolactone. The safety and effectiveness of concurrent therapy of Cardalis™ with pimobendan has not been evaluated. The safety of Cardalis™ has not been evaluated in pregnant, lactating, breeding, or growing dogs. Cardalis™ administration should begin after pulmonary edema is stabilized. Regular monitoring of renal function and serum potassium levels is recommended. Common side effects from a field study include anorexia, vomiting, lethargy, diarrhea and renal insufficiency.
CRD-109-23v2
