Pepper’s Case: A Nonsurgical Approach to Canine Mast Cell Tumors
Sponsored by Virbac
Mast cell tumors (MCTs) are the most common skin tumor in dogs, accounting for ≈20% of all skin tumors.1 MCTs should be promptly removed because of their potential aggressive behavior.
Historically, the only way to remove MCTs has been through surgical excision, which often proves challenging due to tumor location, size, and/or owner aversion to surgery; however, a nonsurgical option has entered the market with STELFONTA® (tigilanol tiglate injection).2
Follow Pepper’s case to learn how STELFONTA provided a safe and effective treatment option.
Pepper’s Case
Pepper, a 10-year-old, neutered male Australian shepherd crossbreed, was presented with a caudal axillary cutaneous MCT measuring 2.5 cm × 1.6 cm × 1.1 cm (total tumor volume, 2.2 cm3) that showed no evidence of metastasis. The veterinarian shared treatment options with his owner, explaining that both surgical excision or STELFONTA injection could treat the tumor.
After discussing that STELFONTA would not require general anesthesia or significant exercise restrictions and that 89% of dogs remain cancer free 1 year after treatment,3 the owner chose this option. In a recent market survey, nearly 2/3 pet owners indicate STELFONTA is the treatment they are most likely to choose after learning its mechanism of action and seeing pictures of dogs being treated.4 They state the fact that STELFONTA eliminates the need of surgery and general anesthesia as the most important reasons for their choice.
STELFONTA
STELFONTA is an FDA-approved treatment for nonmetastatic canine MCTs ≤10 cm3 in volume.5 STELFONTA is an option for cutaneous tumors located anywhere on the body and subcutaneous tumors located at or distal to the hock or elbow.
STELFONTA removes 87% of canine MCTs after 2 treatments and 75% of canine MCTs after a single treatment.6
Administration
Appropriate pre- and post-treatment medications, including a corticosteroid plus blocking agents for both H1 and H2 receptors, must be given to decrease the potential for severe systemic adverse reactions, including death, from mast cell degranulation. Two days prior to the STELFONTA injection, prednisone (0.5 mg/kg PO q12h for 10 days) was initiated. Famotidine (0.5 mg/kg PO q12h for 10 days) and diphenhydramine (2 mg/kg PO q12h for 10 days) were also prescribed at this time. These medications must be started by the day of STELFONTA administration and continued 8 days postinjection to reduce the risk for systemic effects associated with mast cell degranulation. Additionally, gabapentin (10 mg/kg PO q8h for 10 days) was prescribed for pain relief.
On the day of treatment, 1.1 mL of STELFONTA (0.5 mL/cm3 of tumor volume) was injected into the tumor using a fanning pattern.
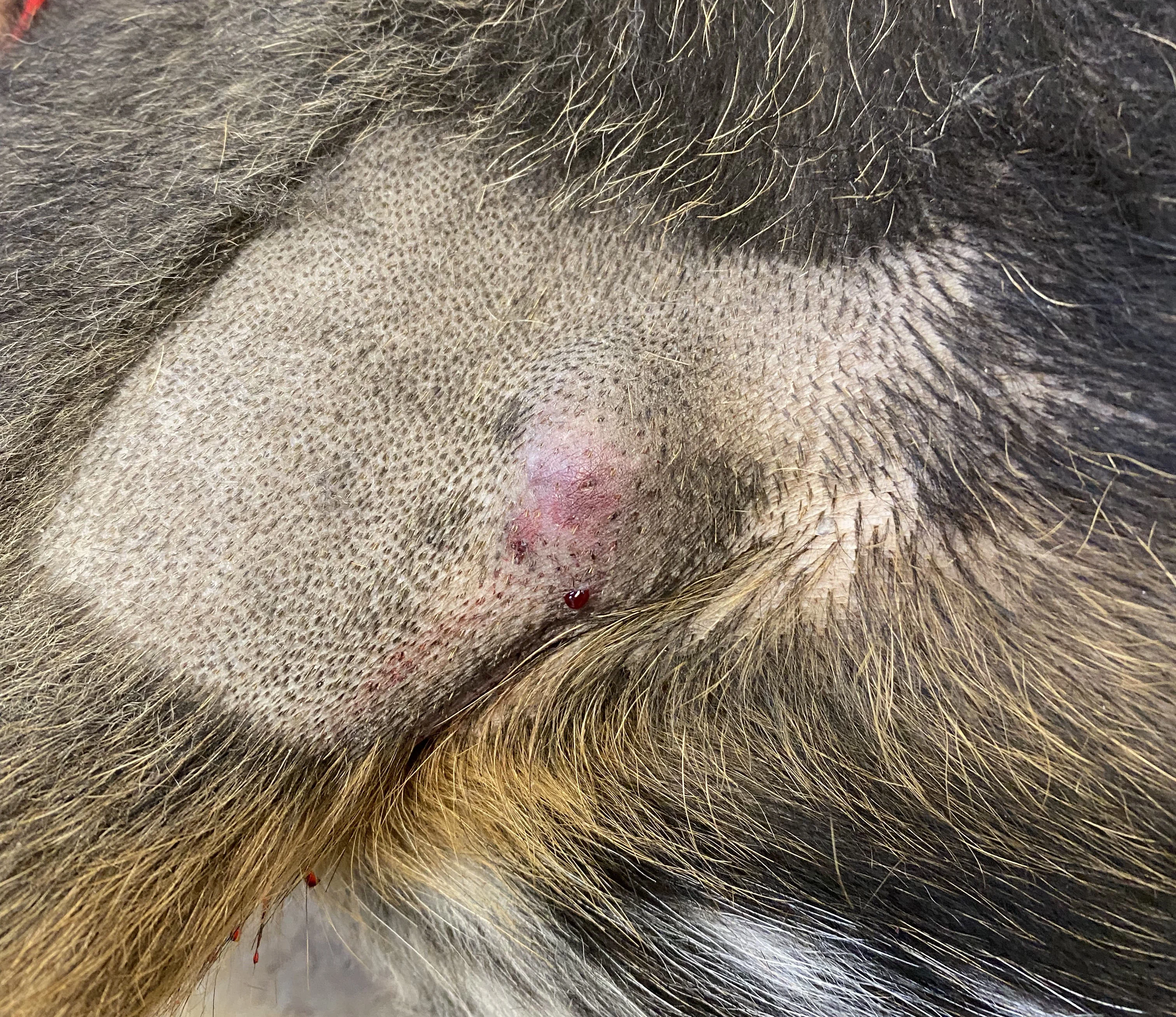
Tumor appearance postinjection
Pepper’s owner was advised that an acute inflammatory response (eg, pain, bruising, swelling) was to be expected in the following days.5 The owner was also advised to monitor for signs of mast cell degranulation (ie, vomiting, diarrhea, tachypnea, tachycardia, weakness, lethargy) over the next 3 to 7 days and to contact the veterinarian if they noticed any of these signs.
Response to Treatment
Pepper returned for a recheck examination 7 days postinjection. The area of tumor destruction appeared to be progressing as expected. Tumor sites are expected to heal by second intention, with most tumors being fully healed in 4 to 6 weeks.7
Pepper’s owner also shared her surprise with how well he was doing posttreatment. After showing mild signs of discomfort for the first 3 days after injection, Pepper returned to normal by day 4. In the following weeks, Pepper’s tumor site was monitored via in-person visits and emails.
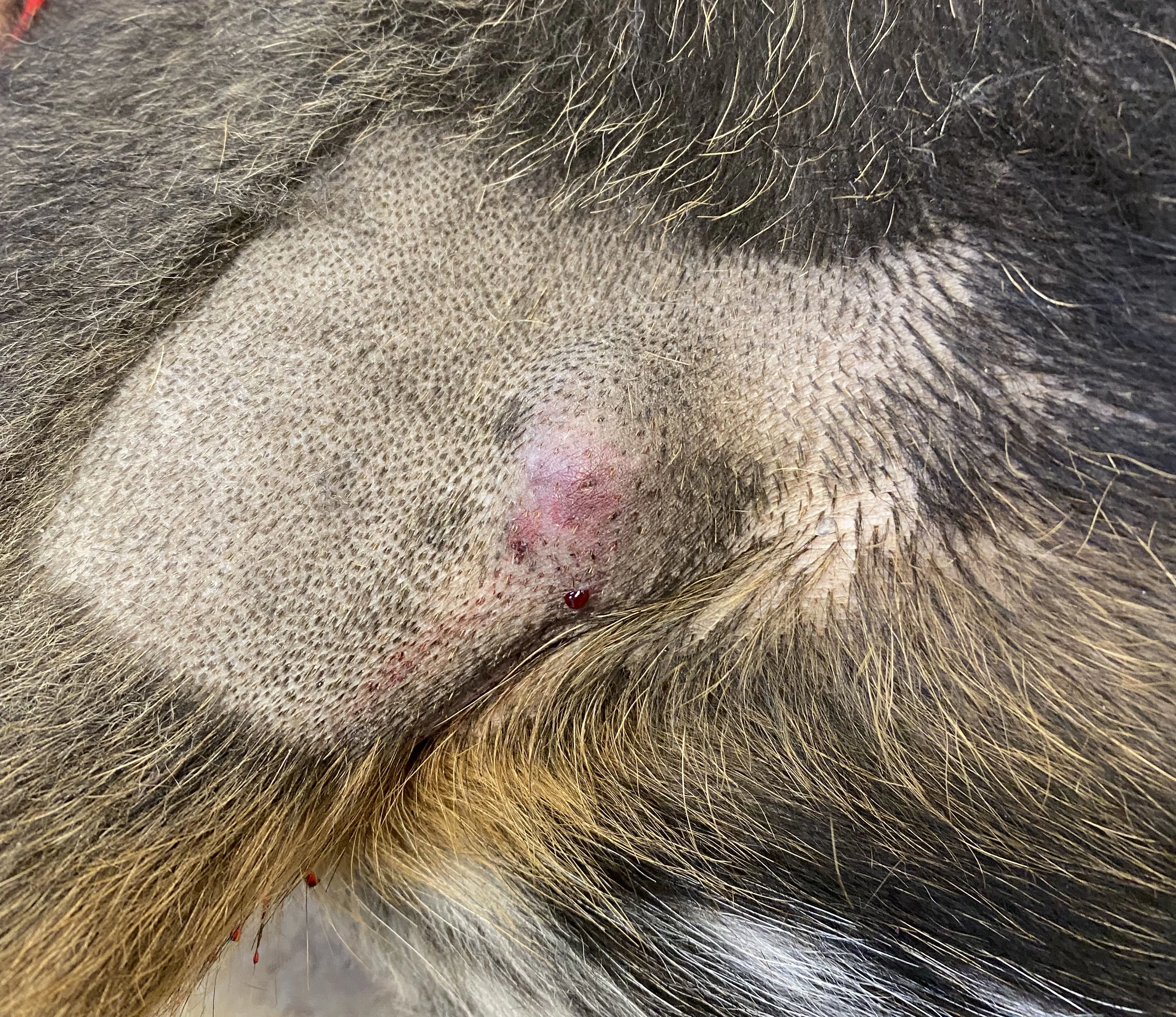
FIGURE 1
Tumor appearance postinjection
Seventy days after STELFONTA injection, Pepper was presented for a wellness examination. The tumor site was fully healed, and Pepper’s owner was advised that no further treatment or monitoring was indicated. Although 75% of dogs treated with STELFONTA typically have a full clinical response to an initial injection,6 as Pepper did, a second injection would be indicated if the tumor had not resolved.
Pepper’s owner was relieved that the MCT had been destroyed and stated it was “remarkable how fast it healed. I’m so glad you thought Pepper was a good candidate for STELFONTA.”
FROM PEPPER’S VETERINARIAN
"I have found STELFONTA to be a product that engages clients. They become excited watching it work before their eyes. It is one of the only products that mitigates some of the fear following a cancer diagnosis. I have and will continue to recommend STELFONTA for MCTs that fall within the usage guidelines."
Conclusion
STELFONTA is a unique, nonsurgical approach to the treatment of canine MCTs and is available for use in a general practice or specialist setting. It provides resolution of MCTs without the need for general anesthesia or surgery, meeting clients’ desires for a safe, effective treatment modality. In the words of Pepper’s owner, "it really is a game changer."
For full prescribing information, contact Virbac at 1-800-338-3659 or visit vet-us.virbac.com/stelfonta.
IMPORTANT SAFETY INFORMATION
Accidental self-injection of STELFONTA® (tigilanol tiglate injection) may cause severe wound formation. To decrease the risk of accidental self-injection, sedation of the dog may be necessary. In dogs, do not inject STELFONTA into subcutaneous mast cell tumors located above the elbow or hock. Formation of wounds, possibly extensive, is an intended and likely response to treatment with STELFONTA along with associated swelling, bruising and pain; these wounds are expected to heal. Appropriate pre- and post-treatment medications must be given, including a corticosteroid plus blocking agents for both H1 and H2 receptors, in order to decrease the potential for severe systemic adverse reactions, including death, from mast cell degranulation. For full prescribing information, contact VIRBAC at 1-800-338-3659 or visit vet-us.virbac.com/stelfontatelfonta.
