Top 5 Gastrointestinal & Hepatobiliary Antibiotics
Craig B. Webb, PhD, DVM, DACVIM (Small Animal), Colorado State University
Antibiotic use in GI and hepatobiliary disease should be judicious, purposeful, and, ideally, directed by culture and susceptibility results when appropriate. These conditions are often chronic or recurring, and repeated courses of ineffective or unnecessary antibiotic “trials” could result in the emergence of antibiotic-resistant organisms. Injudicious use of common antibiotics can also be associated with significant alterations in the microbiota, risking both immediate and far-reaching deleterious consequences.1
The author considers enrofloxacin, metronidazole, and tylosin to be the antibiotics most deserving of thoughtful consideration in the treatment of GI conditions in dogs and cats. Metronidazole and enrofloxacin are also frequently used in the treatment of hepatic disease. Amoxicillin–clavulanic acid and neomycin are additional important antibiotics for pharmacotherapy of hepatobiliary conditions.
1. Enrofloxacin
Enrofloxacin is a fluoroquinolone with broad-spectrum bactericidal activity against aerobic bacteria. Its mechanism of action is believed to be inhibition of bacterial DNA-gyrase, which then prevents DNA supercoiling and synthesis.2 Enrofloxacin also has efficacy against Escherichia coli.
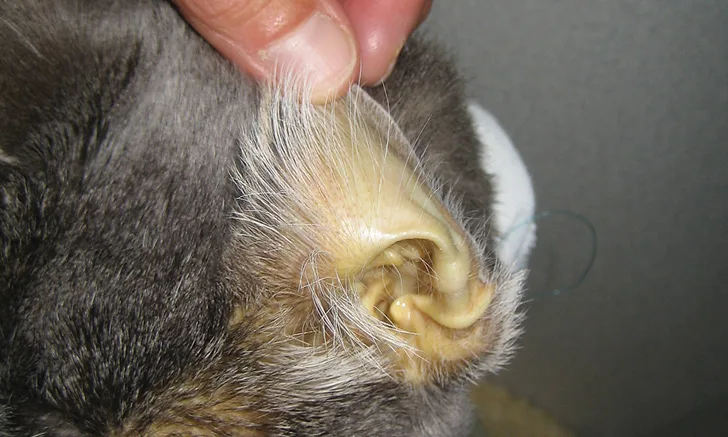
Enrofloxacin represents a unique collective effort of the veterinary profession to successfully identify a specific therapy targeting a defined condition (ie, histiocytic ulcerative colitis [HUC; Figure 1] in boxers) that previously had been almost always terminal. This accomplishment serves as an example of the effort that should inform all areas of antibiotic use in small animal medicine,3-6 particularly because of the therapeutic difficulties now presented by antibiotic resistance. Before enrofloxacin was identified as an effective treatment for HUC, most boxers with this disease were euthanized because immunosuppressive therapy was unable to slow the rapid progression of the condition. Invasive E coli was identified as the causal agent, and remission was correlated with eradication of intramucosal E coli organisms following treatment with enrofloxacin (5-10 mg/kg PO q24h for 6-8 weeks).7-9 Cases of HUC in which enrofloxacin was a critical component of successful therapy have also been reported in French bulldogs, an English bulldog, and several other nonboxer breeds.10-12 It is crucial to note that treating dogs with enrofloxacin before obtaining a definitive diagnosis of HUC has been associated with antimicrobial resistance and a poor clinical outcome.13
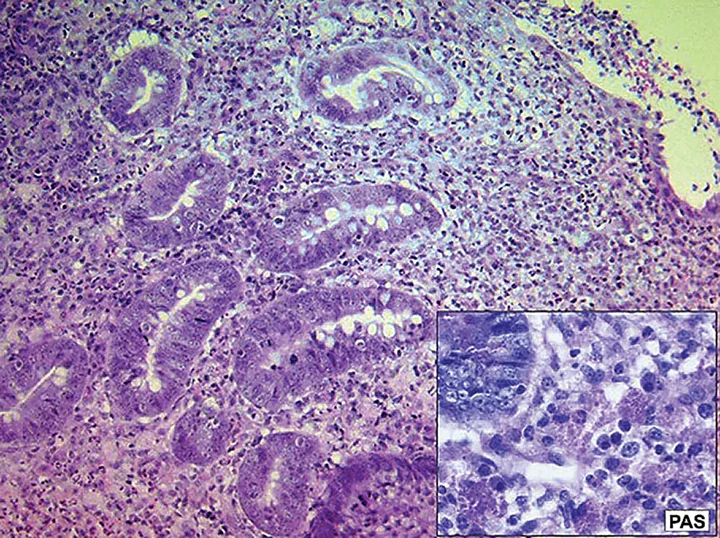
Histopathology and Periodic Acid-Schiff (PAS) stain of a sample obtained from a dog with histiocytic ulcerative colitis
E coli is one of many potential enteropathogenic bacteria associated with GI disease in dogs and cats; however, according to the ACVIM Consensus Statement on enteropathogenic bacteria, the disease is uncomplicated and self-limiting in most cases, molecular identification is sophisticated but causality is extremely elusive, and the injudicious use of antibiotics in GI disease may cause more harm than benefit.14
The most common adverse effects of enrofloxacin are GI in nature, including vomiting, diarrhea, and loss of appetite. Enrofloxacin should not be used in young, growing dogs because of the risk for cartilage damage. Enrofloxacin should be avoided in patients with seizure disorders and used at a reduced dose in patients with a significant loss of renal or hepatic function. Enrofloxacin should not be used at dosages greater than 5 mg/kg/day in cats because of the retinotoxicity reported in this species.2
2. Metronidazole
Metronidazole is a bactericidal antibiotic that targets anaerobes; it is also an antiprotozoal agent used frequently to treat Giardia spp infection. It is one of the most common nonspecific antidiarrheal drugs prescribed in veterinary medicine.15,16 Metronidazole has been shown to have immunomodulatory effects in mice (in vitro) and in humans, and these properties often are cited as justification for its use as a nonspecific GI immunosuppressive agent.17-19 However, recent work in a model of sepsis failed to identify any impact of metronidazole on systemic innate immune responses in humans.20
The veterinary profession’s understanding and appreciation for the importance of the microbiota for GI health and immune function is increasing, along with concern over the untargeted use of antibiotics and their potential for contributing to dysbiosis, and metronidazole is a key component of this discussion. Metronidazole (12.5 mg/kg PO q12h for 14 days) has been shown to reduce some pathogenic bacteria (eg, fusobacteria) and increase some beneficial bacteria (eg, bifidobacteria) but also significantly decrease the bacterial composition and diversity of the microbiota of healthy dogs.21 Composition and diversity are thought to be central components of a healthy microbiome. Recent studies have demonstrated significant dysbiosis and decreased microbiota diversity in dogs with both acute diarrhea and inflammatory bowel disease.22,23
Metronidazole has been used in the treatment of a number of veterinary diseases but most often in combination with other therapeutic agents, making it difficult to assess the efficacy of the antibiotic alone. Metronidazole (15-20 mg/kg PO q12h for 7-17 days) was found to effectively resolve diarrhea in an outbreak of Clostridium difficile-toxin–positive dogs at a small animal teaching hospital.24 In a recent report, metronidazole (10 mg/kg PO q12h for 5 days) was used in combination with trimethoprim–sulfamethoxazole in Labrador retriever puppies presented for anorexia, hematemesis, and hematochezia and diagnosed with Isospora spp infection. All puppies recovered completely.25 In another report, metronidazole (10-15 mg/kg PO q12h for 6 weeks) was used in combination with prednisolone in a group of dogs presented for diarrhea and vomiting with hypoalbuminemia and hypocobalaminemia. Colonoscopy and histopathology showed lipogranulomatous lymphangitis, and fluorescent in situ hybridization (FISH) analysis disclosed invasive bacteria in 20% of the cases. Remission was achieved in 8 of the 10 dogs.26 Another paper, however, reported that combining metronidazole (10 mg/kg PO) with prednisone was no more effective as induction therapy for dogs with inflammatory bowel disease than using prednisone alone.27 A 2007 paper described the use of metronidazole (10 mg/kg PO q12h for 2 weeks) as part of a triple antimicrobial therapy in dogs with chronic vomiting and gastric Helicobacter spp infection. Vomiting frequency was reduced by 86%.28
Exposure to antibiotics, including metronidazole or fluoroquinolones, markedly increases the risk for new-onset inflammatory bowel disease, specifically Crohn’s disease, especially in children.29 Of increasing concern is the emergence of metronidazole-resistant organisms,30 as was demonstrated in one study that found metronidazole-resistant C difficile isolates in dogs with GI disorders.31 Metronidazole is frequently prescribed for treatment of hepatic encephalopathy in dogs and cats, but controlled clinical trials evaluating the effectiveness of this therapy in veterinary patients have not been conducted.32
3. Tylosin
Tylosin is a macrolide antibiotic thought to bind 50S ribosomes and inhibit protein synthesis. It is frequently used as adjunct treatment for diarrhea in veterinary patients with a variety of diseases. A specific GI condition in dogs termed tylosin-responsive diarrhea has been identified in middle-aged, large-breed dogs with chronic diarrhea that resolves rapidly following tylosin treatment but returns just as quickly with the cessation of tylosin treatment.33 A subsequent study found that the combination of tylosin (20 mg/kg PO q24h for 10 days) and a highly digestible canned food diet effectively controlled chronic diarrhea for at least 3 months in a group of beagles.34 In a randomized placebo-controlled clinical trial in dogs, recurrent diarrhea that had previously resolved with tylosin treatment responded to tylosin again in 85% of cases (25 mg/kg PO q24h for 7 days).35 Later work suggested that a dose as low as 5 mg/kg PO q24h for 7 days may be just as effective in cases of relapsing diarrhea.36 In a translational study, a 10-day course of tylosin significantly improved diarrhea, decreased colonic inflammation, and decreased serum C-reactive protein levels in rhesus macaques with chronic diarrhea.37
Tylosin (20-22 mg/kg PO q24h for 14 days) may resolve diarrhea because it increases concentrations of Enterococcus spp- and E coli-like organisms while decreasing fusobacteria and Bacteroidales in the jejunum of healthy dogs.38 A study of dogs with recurrent tylosin-responsive diarrhea found that tylosin administration significantly increased Enterococcus spp in those dogs with diarrhea that resolved following administration of tylosin (25 mg/kg PO q24h for 7 days).39
Like metronidazole, tylosin is often used for properties believed to be immunomodulatory. Although tylosin has not been shown to demonstrate such properties, studies of the macrolide antibiotic tilmicosin in large animals suggest that it has immunomodulatory and anti-inflammatory properties.40 Tylosin is one of the most frequently prescribed medications for dogs with inflammatory bowel disease.41
4. Amoxicillin–Clavulanic Acid
Amoxicillin–clavulanic acid is a bactericidal aminopenicillin with a β-lactamase inhibitor. Penicillins act by inhibiting cell wall synthesis. Adverse effects are rarely serious, although GI signs (eg, anorexia, vomiting, diarrhea) are relatively common.
Studies published in 2007 and 2016 demonstrated that bacterial cholangitis and cholecystitis (Table), although rarely reported in the literature, should be considered in dogs and cats presented for vomiting, jaundice, and abdominal pain and/or fever.42,43 These patients commonly have an increase in liver enzyme activity, hyperbilirubinemia, an inflammatory leukogram, and ultrasonographic evidence of gallbladder abnormalities. Because antimicrobial resistance has become common with aerobic isolates, antibiotic choice should be based on culture and susceptibility testing. Sampling the bile or gallbladder wall—not the liver—results in the greatest yield for a positive culture. In these studies, metronidazole was used to treat most anaerobic infections, whereas a fluoroquinolone (eg, enrofloxacin, ciprofloxacin) or amoxicillin–clavulanic acid was used to treat most patients with aerobic infection. In the absence of antimicrobial susceptibility testing, treatment with a broad-spectrum antibiotic is indicated and may require combination of a fluoroquinolone, penicillin, and metronidazole or a fluoroquinolone and amoxicillin–clavulanic acid. Use of empiric therapy may fail because of the prevalence of antimicrobial resistance in the common isolates.42,43
Table: Common Bacteria Causing Bacterial Cholangitis in Dogs & Cats
According to the WSAVA International Liver Standardization Group, feline cholangitis is the predominant liver disease in cats after hepatic lipidosis.44 In cases of acute neutrophilic cholangitis, a bacterial infection is likely the inciting cause, and many of these cats are presented with significant clinical morbidity.45 Clinical signs include acute onset of vomiting, diarrhea, anorexia, and lethargy, and patients may be febrile and icteric (Figure 2). These cats may have pancreatitis and gastroenteritis as concurrent conditions (feline triaditis); aggressive supportive care along with directed antibiotic therapy may be critical to a successful outcome. Gallbladder aspiration for cytology (Figure 3) and culture has become the diagnostic test of choice in these cases, and culture and susceptibility results should dictate the choice of antibiotic.46,47 The bacteria most frequently identified in feline neutrophilic cholangitis are enteric organisms (Figure 4), including E coli, Enterococcus spp, Bacteroides spp, Clostridium spp, and Streptococcus spp.48 In lieu of culture and susceptibility results, empiric therapy with amoxicillin–clavulanic acid for 8 weeks is recommended.49
FIGURE 2 Icterus in a cat with acute neutrophilic cholangitis
5. Neomycin
An important potential clinical consequence of decreased liver function is hepatic encephalopathy. In dogs and cats, hepatic encephalopathy is most commonly associated with portosystemic shunts (congenital or acquired), portal hypertension or liver cirrhosis (eg, from chronic copper hepatopathy), and, more rarely, acute hepatic failure (eg, from drugs and toxins). Although the pathogenesis is not completely understood, intestinal bacteria are thought to represent an important source of ammonia production, and increased systemic ammonia concentrations play a central role in this condition.50
Urease-producing bacteria in the GI tract convert urea to ammonia. One treatment strategy uses antibiotics to change the GI microbiota in a way that decreases bacterial production of ammonia. The importance of the microbiota to ammonia production was highlighted by a study showing that probiotic therapy resulted in a significant reduction in overt encephalopathy in humans with liver cirrhosis.51
Neomycin is a bactericidal aminoglycoside that binds to the 70S ribosomal subunit. In dogs and cats, oral neomycin (20 mg/kg PO q8-12h) is still used in conjunction with lactulose for treating hepatic encephalopathy, although, as in humans, both nephrotoxicity and ototoxicity are serious concerns. Metronidazole (7.5 mg/kg PO q8h or q12h) has been used as an alternative antibiotic in veterinary patients.2
HUC = histiocytic ulcerative colitis