Tumor Grading & Staging in Dogs
Francesca Lerner, DVM, University of Florida
Stacey Fox-Alvarez, DVM, MPH, DACVIM (Oncology), University of Florida, Community Care Veterinary Specialists, Gainesville, Florida
Tumor grading and staging can help guide clinical decision-making. Grading uses histopathology to provide information on the anticipated biologic behavior of a tumor. Staging is performed after initial diagnosis to assess the extent of a tumor throughout the body. Staging schemes provide guidance on potential sites of metastasis.
It is important to understand tumor grading and staging (see Tumor Grade vs Stage) for directing further diagnostics, guiding treatment options, and elucidating prognosis for common cancers seen in veterinary patients.
Tumor Grade vs Stage
Grade
Derived from histopathologic analysis of biopsy
May be predictive of behavior, depending on tumor type
Helps answer the question of what
Stage
Describes the extent of a tumor, often accounting for tumor size and presence of local and distant metastasis
Helps answer the question of where
Grading
Tumor grading relies on microscopic characteristics of tissue samples assessed via histopathology. Presence, absence, and/or degree of tumor-specific characteristics are assessed, and a grade is assigned according to published schemes.<sup1 sup>
Grading criteria vary for each tumor type but commonly include mitotic index (eg, number of cells dividing in 10 high-power microscopic fields2), degree of differentiation (ie, how closely cells resemble those found in normal tissue), degree of necrosis (indicator of outpacing resource availability), cellular pleomorphism/atypia (ie, variability of individual tumor cells compared with each other and with normal cells), and degree of invasiveness.1
Grade generally increases as tumor characteristics deviate further from normal tissue appearance. Depending on tumor type, increasing grade may also correlate with more aggressive anticipated behavior. For many tumors (eg, mast cell tumor, soft tissue sarcoma, pulmonary and mammary carcinoma), grade is predictive of metastasis and/or recurrence and should therefore impact recommendations for additional diagnostics (eg, staging) and treatment (eg, chemotherapy, scar revision surgery, radiation).1
When possible, surgical biopsies should be submitted for histopathologic evaluation to more accurately establish grade and guide clinical decision-making, particularly when diagnosis and grade may influence pet owner decisions.1
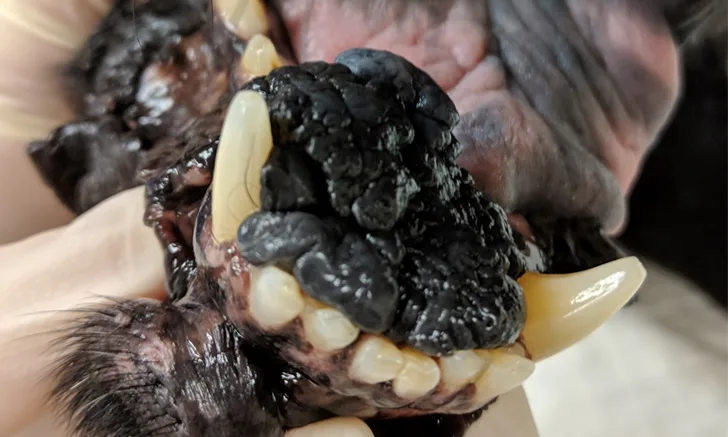
Cutaneous Mast Cell Tumor
Mast cell tumors (MCTs) are common skin tumors in dogs (Figure 1) that can exhibit a variety of biological behavior, emphasizing the importance of grading after surgical excision.
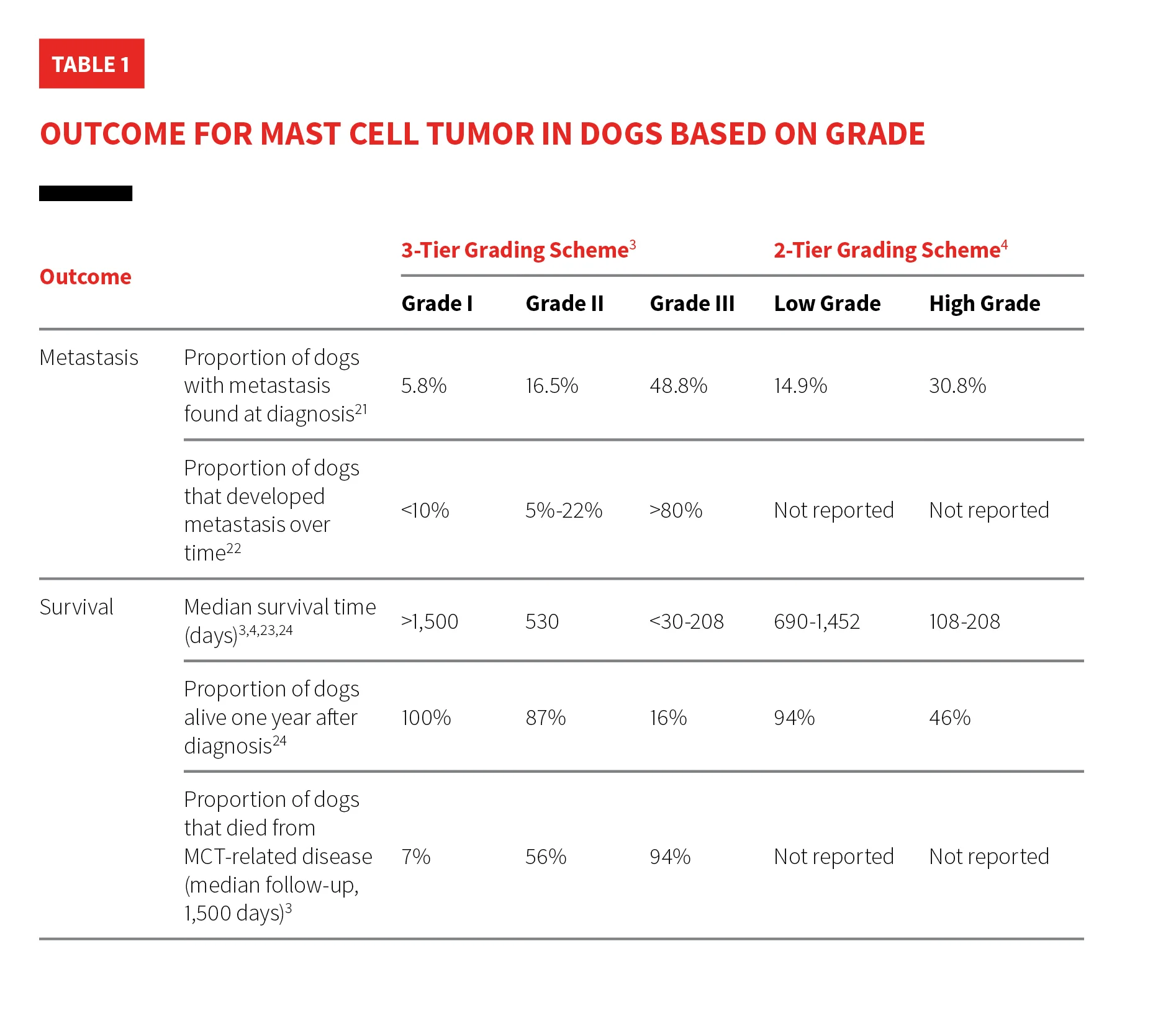
There are many grading schemes for MCTs, but the Patnaik 3-tier and Kiupel 2-tier systems are commonly used by pathologists (Table 1). The Patnaik system classifies MCTs as 1 of 3 grades.3 The Kiupel system categorizes MCTs as either low or high grade and is commonly used to provide prognostic information for tumors classified as Patnaik grade II.3-5
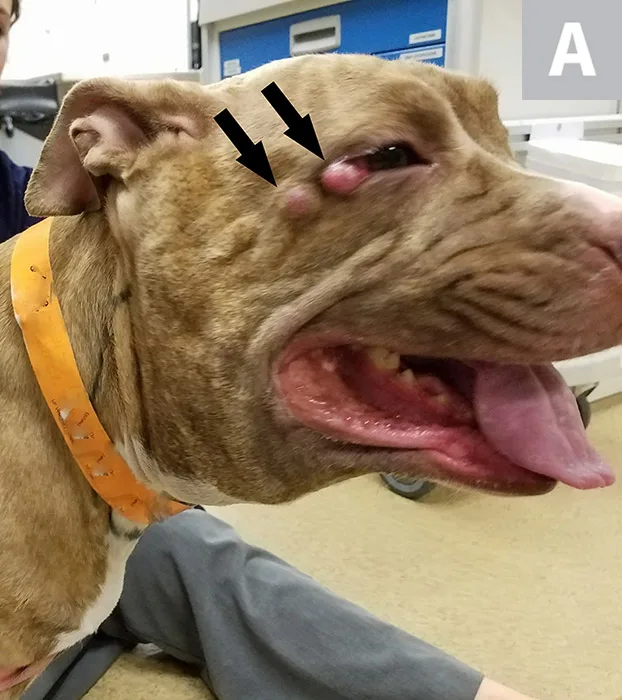
FIGURE 1A
Right and left lateral views of a dog with mast cell disease. Multiple tumors in the same region indicate stage III mast cell disease based on WHO clinical stage criteria. Two small dermal MCTs (A; arrows) can be seen adjacent to the right eye. Marked regional lymphadenopathy secondary to metastasis of mast cell disease is present. These lymph nodes coalesce with swollen local tissues to form an extensive subcutaneous mass effect on palpation (B and C; arrows).
Soft Tissue Sarcoma
Soft tissue sarcomas are common cutaneous/subcutaneous tumors in dogs; grade should influence prognosis and treatment decisions.6 Tumor types commonly include fibrosarcoma, peripheral nerve sheath tumor, myxosarcoma, liposarcoma, and malignant mesenchymoma.7 Soft tissue sarcomas are graded using a scheme (Table 2); data regarding the impact of tumor grade on outcome are available (Table 3).8

Table 3: Outcome for Soft Tissue Sarcoma in Dogs Based on Grade
Staging
Tumor stage describes the distribution of disease in the body. Staging is the act of performing diagnostic tests to systematically evaluate the spread of cancer to determine tumor stage and is typically performed as a baseline prior to initiating treatment in cancer patients. Staging can be repeated over time in patients at risk for tumor spread or with known spread to monitor change from baseline, assess rate of change, and determine response to treatment.
Understanding the inherent biologic behavior of common tumor types is key when prioritizing diagnostic testing for staging. Tumors can display a range of behaviors in each category and patient and do not always follow the typical pattern of metastasis (Table 4).
Table 4: Tumor Types & Initial Staging Recommendations1
Many tumor types have unique staging schemes devised by the World Health Organization (WHO).9 The tumor–node–metastasis (TNM) system indicates distribution of disease in relation to sites at risk for tumor involvement and reports the status of tumor size or extent as T1 to T4. For some tumor types, stage is influenced by increasing tumor size; specific size designations are incorporated in the staging schemes. Lymph node metastasis is reported as absent (N0), regional (N1), or distant (N2). Distant metastasis is reported as absent (M0) or present (M1). Schemes for some tumor types account for the presence or absence of systemic signs caused by the tumor, designated as substage b (Figure 2) or substage a, respectively. Substage is determined separately from and reported immediately following stage.9
When determining TNM stage, tumor (T), node (N), and metastasis (M) should be assessed individually based on tumor characteristics and the highest applicable stage selected. For example, a tumor categorized as stage II based on size but stage III based on distant metastasis would be stage III. Increases in stage occur with larger tumor size, regional/distant metastasis, and, in some cases, presence of systemic signs caused by disease that often correlate with poor outcomes.1,9
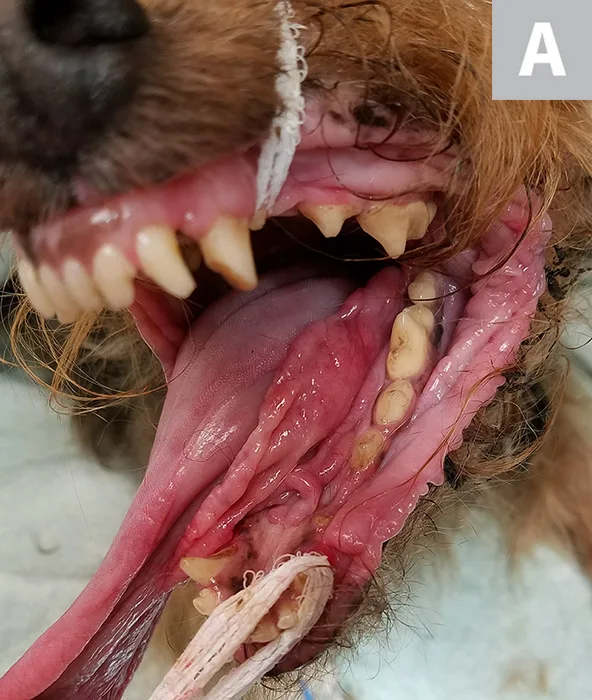
Stage Vb, generalized (multicentric) lymphoma with oral cavity involvement in a systemically ill dog that exhibited generalized lymphadenopathy. Marked edema and erythema of the sublingual tissue (A) and the tonsils and pharyngeal area (B) were secondary to lymphoma.
WHO staging schemes are available for several common tumor types in dogs (Table 5). For example, a dog with a single cutaneous MCT with nodal and distant metastasis without systemic signs should be categorized as stage IVa; a dog with a 5-cm oral melanoma without evidence of metastasis should be categorized as T3N0M0 or stage III.
Osteosarcoma
Osteosarcoma is a mesenchymal tumor arising from bone that typically exhibits a standard metastatic pattern. Although <15% of patients with osteosarcoma are presented with radiographic signs of metastasis, ≈90% die from cancer spread (predominantly to the lungs) within one year when amputation is pursued as the sole treatment.10,11
Staging dogs with osteosarcoma should include 3-view inspiratory thoracic radiography because metastasis greatly impacts survival and may influence the owner’s decision to amputate.12 Amputation alone in dogs with osteosarcoma without metastasis at time of diagnosis resulted in a median survival of 134 days.11 Survival is shortened to a median of 59 days once pulmonary metastatic disease is diagnosed.13 Thorough physical examination to guide further diagnostics is warranted. Abnormal lymph nodes should be sampled because lymph node metastasis is rare but predicts shortened survival (median, 59 days).14 Bone pain distant from the primary tumor and abnormalities palpated in the abdomen should be investigated to evaluate for bone or visceral metastasis or concurrent disease.12
Hemangiosarcoma
Splenic hemangiosarcoma in dogs illustrates the importance of the TNM staging scheme and its impact on patient prognosis.
Anecdotally, small, nonruptured splenic masses without metastasis (stage I) do not typically cause clinical signs and are often incidentally found in patients undergoing imaging for reasons unrelated to hemangiosarcoma. Patients with splenic hemangiosarcoma are more commonly presented emergently due to a ruptured splenic mass with or without metastatic disease (stage III or stage II, respectively). Thoracic radiography is indicated to assess for overt pulmonary metastasis if hemangiosarcoma is a differential diagnosis. Other diagnostic tests (eg, abdominal ultrasonography, thoracic CT, abdominal CT, echocardiogram) can help preparation for surgery and may reveal evidence of metastatic disease that could affect the owner’s decision to pursue surgery.
Biopsy of any grossly abnormal sections of liver, body wall, omental nodules, or grossly abnormal lymph nodes should be performed during abdominal exploratory to help determine stage. One study evaluating histopathologic findings in patients undergoing splenectomy for hemangiosarcoma found that 0% of grossly normal liver biopsies and 50% of grossly abnormal liver biopsies contained metastasis.15
A study evaluating outcomes in 208 dogs with splenic hemangiosarcoma reported a median survival time of 1.6 months in dogs treated with surgery alone and 3.4 months in dogs treated with any form of chemotherapy in addition to surgery.16 Clinical stage significantly impacted survival times independently of chemotherapy status; postoperative median survival time was 5.5 months in stage I patients, 2 months in stage II patients, and <1 month in stage III patients.16 One year after splenectomy, 35.3% of stage I dogs, 12.5% of stage II dogs, and 0% of stage III dogs had survived.16
Oral Melanoma
TNM staging scheme and patient prognosis are significantly influenced by primary oral melanoma tumor size (Table 5). Size alone can predict survival in dogs treated with surgical removal of the primary tumor.17,18 Patients with tumors <2 cm in diameter (stage I), 2 to 4 cm in diameter (stage II), or >4 cm in diameter (stage III) lived a median of 17 to 18 months, 5 to 6 months, and 3 months, respectively (Figure 3).18 Lymph node metastasis (stage III) conferred a median survival time of 3 months.18 Survival data in patients with pulmonary metastasis (stage IV) are limited; survival of <3 months can be anticipated in the authors’ experience. Thoracic radiography and mandibular lymph node aspiration are the minimum recommended diagnostic tests prior to pursuing curative-intent surgery.19 Lymph node metastasis can be present despite normal lymph node size and feel.20
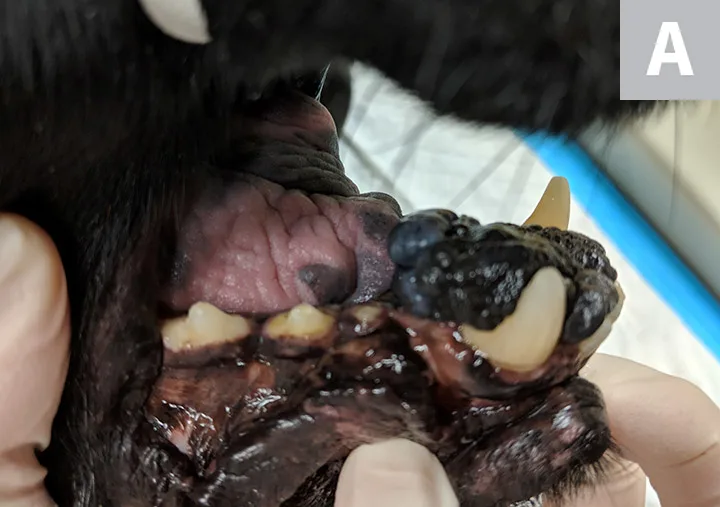
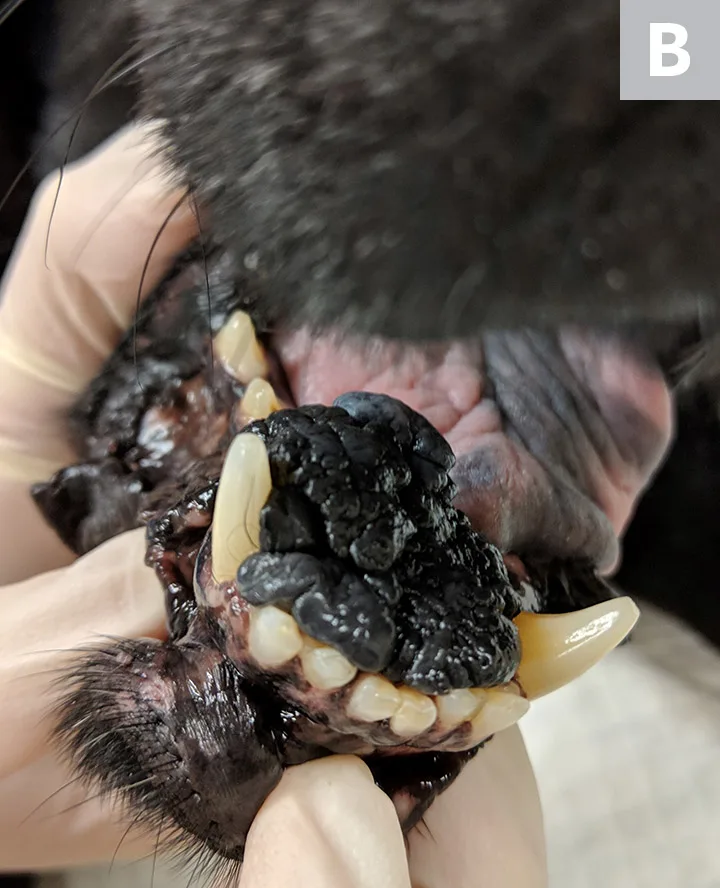
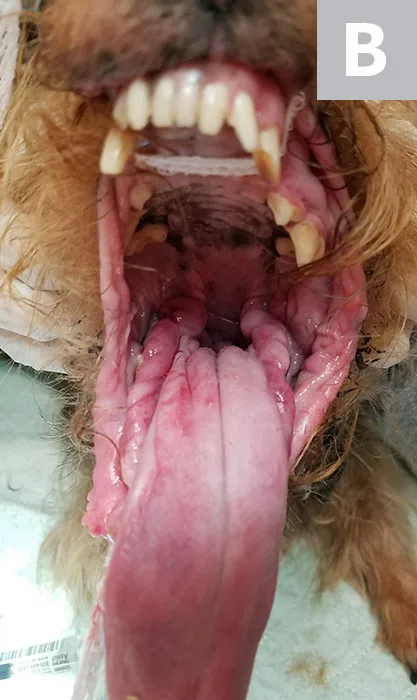
Lateral (A) and rostral (B) views of a dog with oral malignant melanoma measuring >4 cm at its largest diameter. According to the TNM staging scheme, this patient meets the criteria for T3 and stage 3 disease if distant metastasis is not detected.
Conclusion
Tumor grade and stage can impact patient prognosis and influence treatment decisions. Grade should direct recommendations for additional treatment (eg, revision surgery, radiation, chemotherapy) and aid in determining the necessity and frequency of monitoring and staging after a tumor is removed. Choosing staging tests based on the most likely patterns of tumor spread prioritizes tests more likely to influence treatment decisions. This approach can best direct owner finances and may help detect early cancer spread (when intervention has a higher chance of success and quality of life has not yet declined).
Understanding the principles of grading and staging can inform care, but outcomes vary. Prognoses and recommended treatment plans should be based on median survival times and population trends. Numerous factors (eg, paraneoplastic syndromes, comorbidities, immune function, patient signalment) can influence outcome, and some patients may respond favorably to certain treatments, whereas others do not. Owners should be made aware that oncologic treatment is focused on improving and/or extending quality of life and may not be curative.