Most wounds can be successfully managed with standard care (ie, clipping, cleaning, debriding with either suture closure or open wound management with topical dressings and bandage changes); however, infection, mechanism and extent of injury, systemic illness, patient temperament, pet owner compliance, and financial constraints can negatively impact wound healing and patient prognosis.1 In patients with extenuating factors, wound management and closure may require complex techniques.
Case 1: Congenital Musculoskeletal Defect
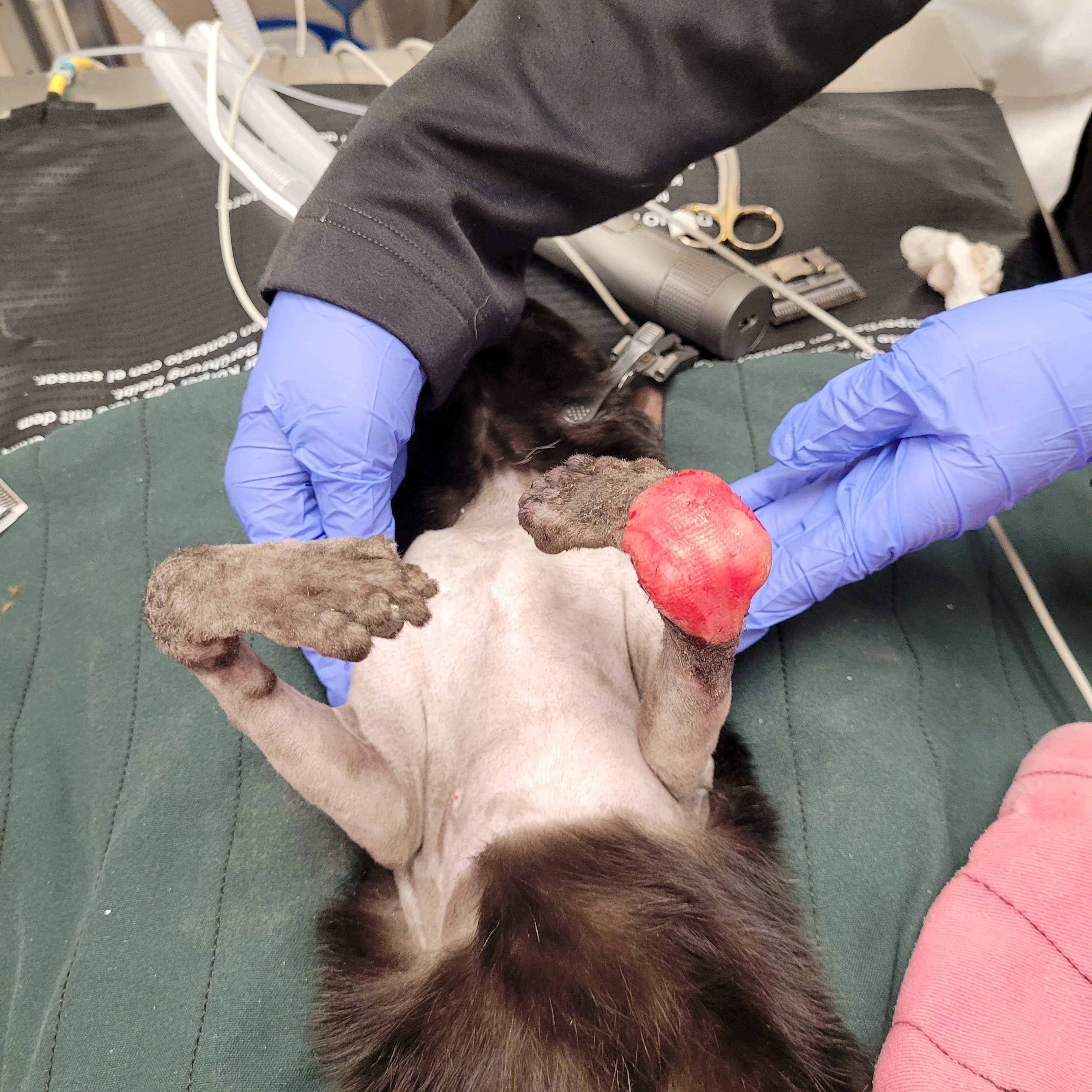
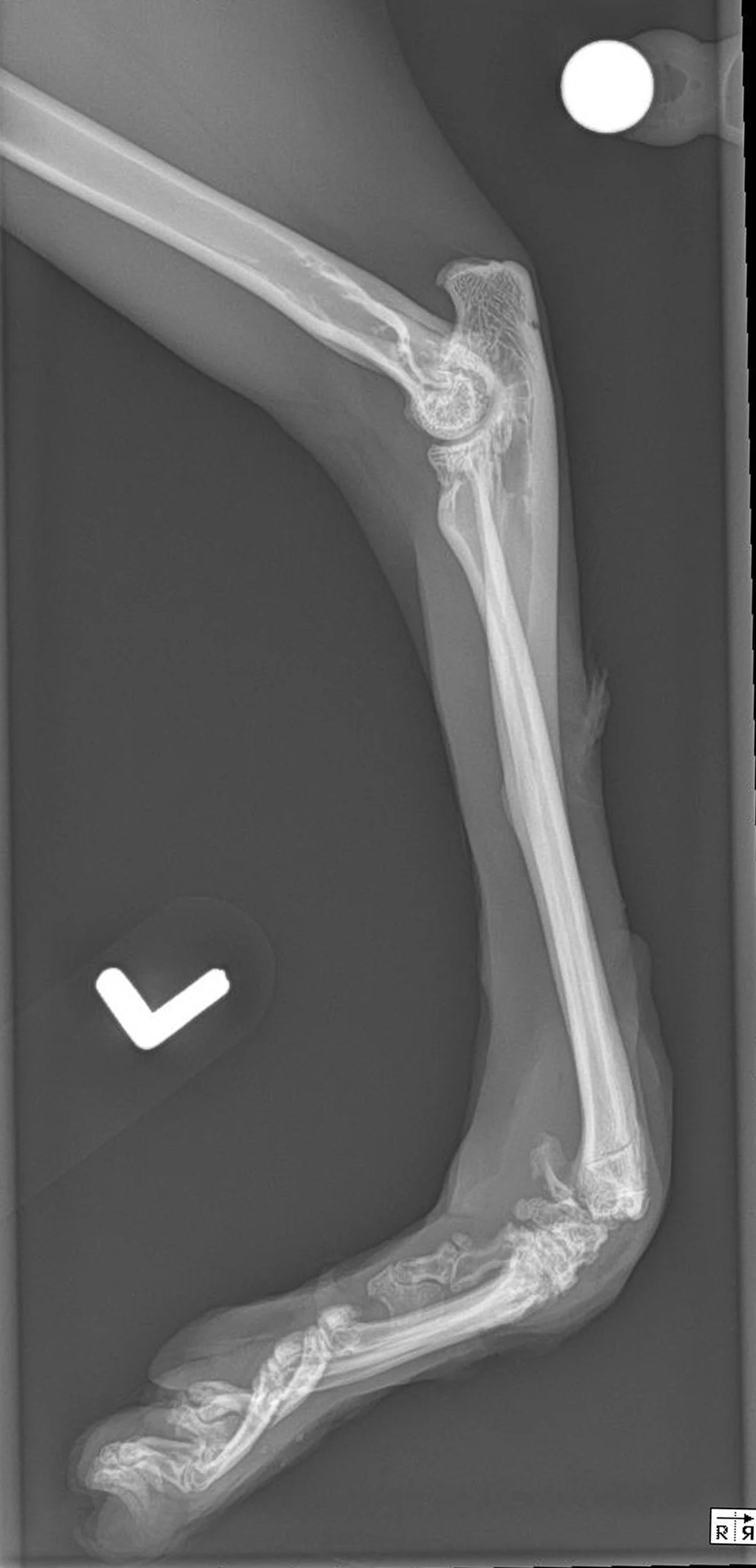
Figure 1 A 2-year-old cat was presented for a congenital musculoskeletal defect that included bilateral hyperflexion of the carpi and 180-degree torsion of the left manus. Chronic weight-bearing had resulted in a thickened left dorsal carpus, lack of epithelium, and fine granulation tissue. Initial treatment included bilateral superficial and deep digital flexor tendon transections, right carpal arthrodesis with plate fixation and postoperative carpal splinting, and left carpal arthrodesis with external skeletal fixation. A skin graft was recommended but declined by the owner. A nonadherent, cuttable, hydrophilic foam dressing, cast padding, and self-adhesive wrap were applied and changed every 3 to 7 days.
Use of a nonadherent foam dressing, petrolatum emulsion coated mesh, or thin silicone sheet as a primary layer can promote contraction and epithelialization of granulated wounds and reduce the risk for damage to delicate tissues during dressing removal.1 Wounds heal best in a moist environment, which facilitates granulation tissue formation, wound contraction, and epithelial cell migration.2 The most appropriate type of dressing depends on the amount of fluid production. For an effusive wound, the dressing should either absorb or allow passage of fluid or exudates so the risk for infection and tissue maceration are reduced.3 Even nonadherent dressings can stick to wound surfaces; softening fibrin and dried exudate with warm saline may facilitate dressing removal.3

Figure 2 Within 4 weeks after external fixator placement, poor owner compliance and urine-soaked bandages resulted in moist dermatitis and superficial pin tract osteitis. Culture and susceptibility testing of the skin following antiseptic preparation was positive for methicillin-resistant Staphylococcus aureus (MRSA). Initial management included daily cleansing of the pin tracts with 0.05% chlorhexidine solution, cleansing of the granulation bed with saline, application of a compounded amikacin spray, and application of a protective wrap over the fixator. Chlorhexidine solution at a concentration of 0.05% exerts an antimicrobial effect without causing tissue injury.4 Systemic antibiotics were not required.
Superficial pin tract infections occur in 9% of cats with external skeletal fixators and often resolve with antimicrobial administration, appropriate local care, or implant removal.5 MRSA colonization rates are 0% to 9% in dogs and cats.6,7 This cat’s owner was a nurse in a health care facility, increasing the risk for MRSA infection.6,7
Until the infection is resolved, owners of pets with MRSA infections should limit contact with their pet, wash their hands frequently, clean the environment frequently, and use gloves when handling the wound or contaminated bandage material.6 Veterinary staff should isolate infected patients, wear personal protective equipment (PPE; eg, gowns, gloves) during bandage changes, designate equipment and materials specifically for use with the patient, and fully clean and disinfect themselves (eg, via thorough hand washing, discarding or washing and sterilizing PPE) and the environment (eg, tables, floors, contact areas) following patient care.6
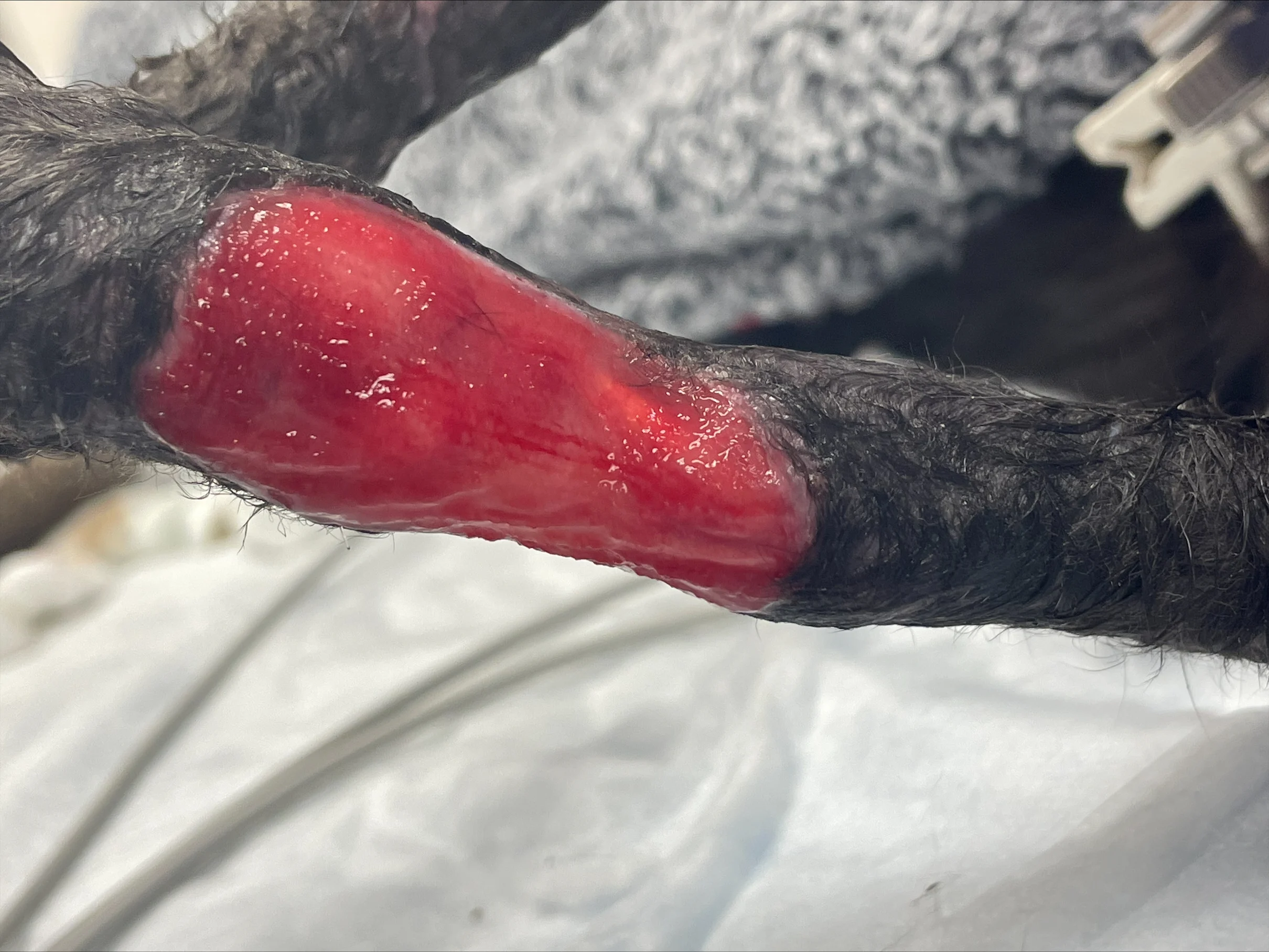
Figure 3 The external fixator was removed 8 weeks after placement, and topical management, including hydrophilic foam on the wound bed to maintain moisture without promoting maceration or infection, was continued.1,3,8 After 11 weeks, the left carpal wound was smaller and contained a healthy granulation bed (fine granular appearance and new marginal epithelium).9 In cats, healing of open wounds is slow, particularly in areas that lack subcutaneous tissues, are under tension, or have poor blood supply from trauma or other causes.9,10 To speed healing in this patient, an autologous, full-thickness mesh graft was collected from the left lateral thorax, applied to the wound, and bandaged with petrolatum-impregnated cellulose acetate mesh dressing, cast padding, caudal splint, and self-adhesive wrap. Petrolatum-impregnated mesh dressings are nonadherent, maintain a moist wound bed, allow drainage (which prevents maceration of surrounding skin), and can be stapled or sutured to healthy skin around the graft to prevent movement during bandage changes.3,11 Cast padding, splint, and self-adhesive wrap were removed and replaced on days 3 and 5 after grafting to allow inspection of the site. Mesh was not removed until day 7.
Bandage changes in the first 5 to 7 days should be performed carefully and with the patient under sedation to avoid disruption of delicate vascular ingrowth.11
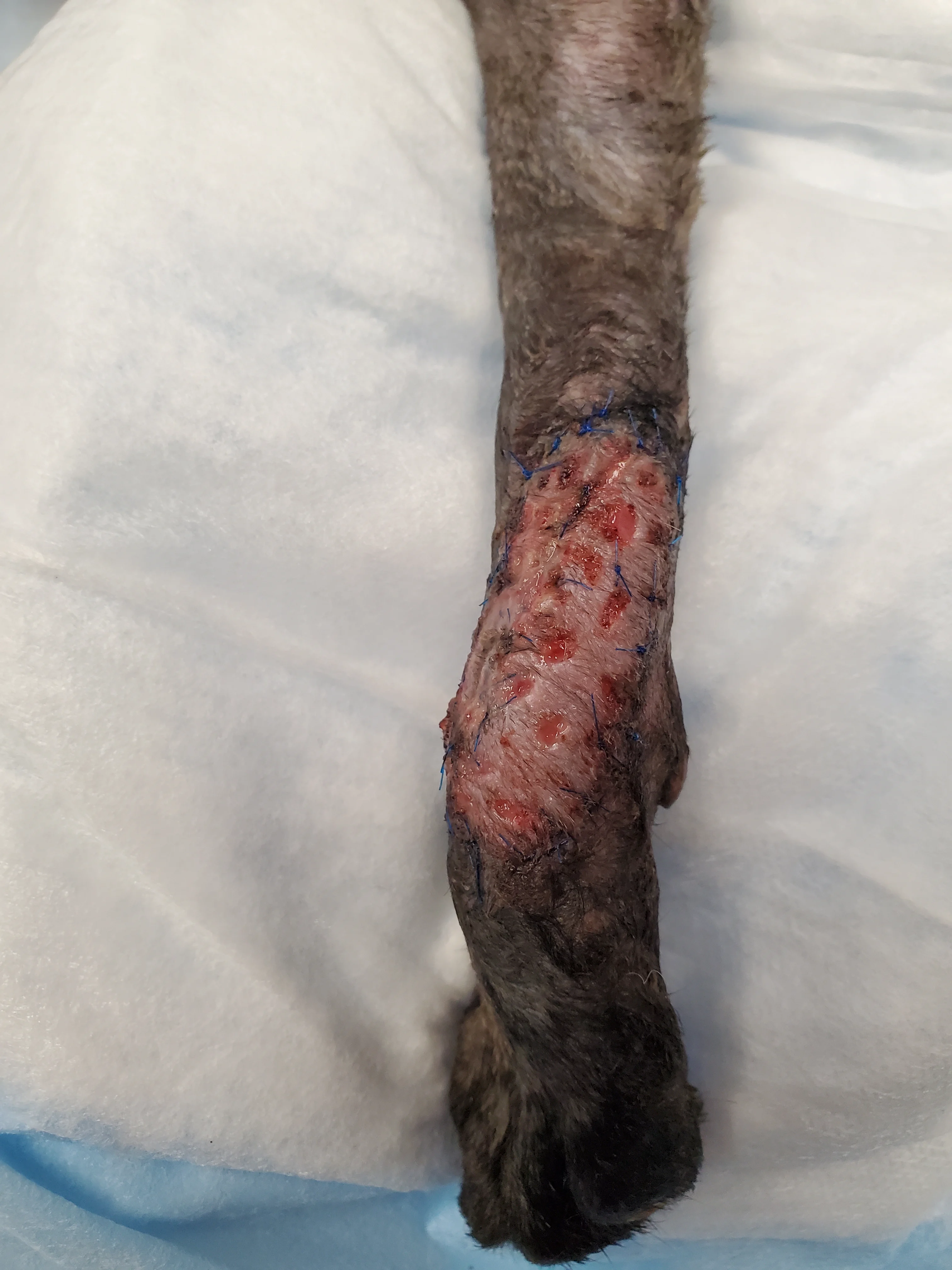
Figure 4 The skin graft appeared pink 5 days after placement, and granulation tissue was visible within the meshing.
Revascularization of mesh grafts is usually evident within 1 week following placement.12 Within the first 3 days, the graft may appear dark red to purple or gray, as the graft survives by imbibing local tissue fluid; however, color gradually improves to pale pink and then pink with vascular ingrowth.11 Discolored grafts should not be removed prematurely because hair follicles and other deep structures may still survive and be a source for new epithelial growth.11 Initially, padded bandages should be placed over the graft for 10 to 14 days as protection from movement or trauma and be changed as infrequently as possible to prevent graft disruption while keeping the site clean. Reinnervation may require an extended time period; therefore, continued protection of the site for several weeks with a bandage, Elizabethan collar, or other device may be necessary to prevent self-trauma in some patients.11,13
Case 2: Degloving Injury
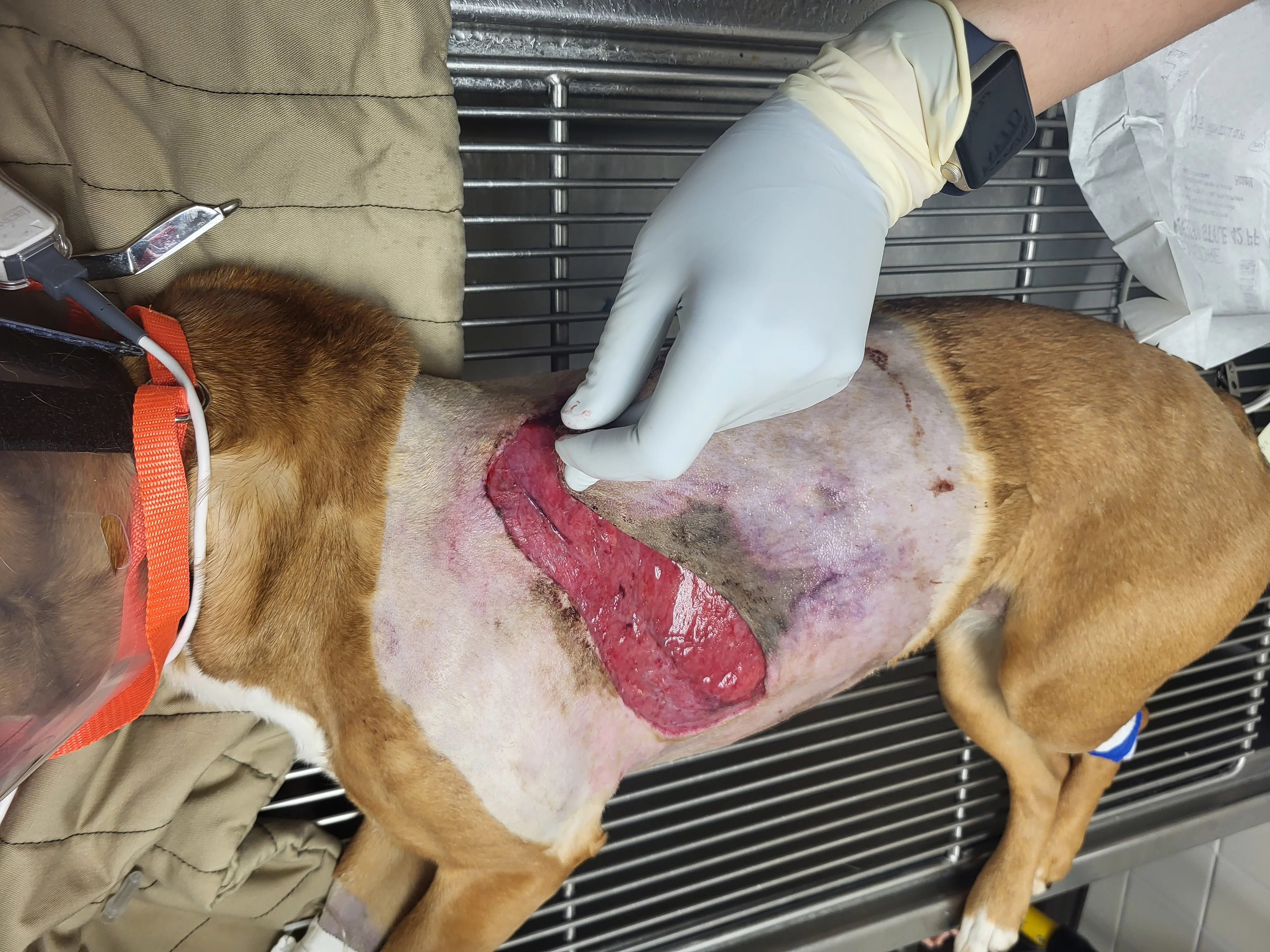
Figure 5 A 4-year-old crossbreed dog was presented for a degloving injury from an unknown source. Following stabilization and radiography, the dog was anesthetized to allow clipping of the fur around the wound, as well as cleansing, lavage, and debridement of the wound. Topical medical-grade manuka honey gel was applied to the open wound bed, which was then covered with laparotomy pads held in place with a soft padded bandage. Ampicillin/sulbactam (30 mg/kg IV every 8 hours), fentanyl (3 μg/kg/hour CRI), and ketamine (0.18 mg/kg/hour CRI) were administered.
For avulsion and degloving injuries, the full extent of tissue damage may not be immediately evident,14 and owners should be counseled that multiple debridement sessions with the patient under anesthesia may be needed and wound management and closure may be a long, complex, and expensive process. Tissue that is clearly nonviable (eg, black, gray, green, leathery) should be removed with sharp dissection during each session. Questionable subcutaneous tissues can be debrided autolytically with dressings such as honey.1 Lavage can be performed with isotonic fluids or tap water; pressures of 5 to 15 pounds per square inch are most suitable for removing debris and/or dead cells from the wound bed while avoiding tissue damage and bacterial seeding.1 This pressure is best achieved by placing a 1-L fluid bag in a pressure cuff, attaching a fluid extension set and a 16- to 22-gauge needle, and pressurizing the bag to 300 mm Hg.4
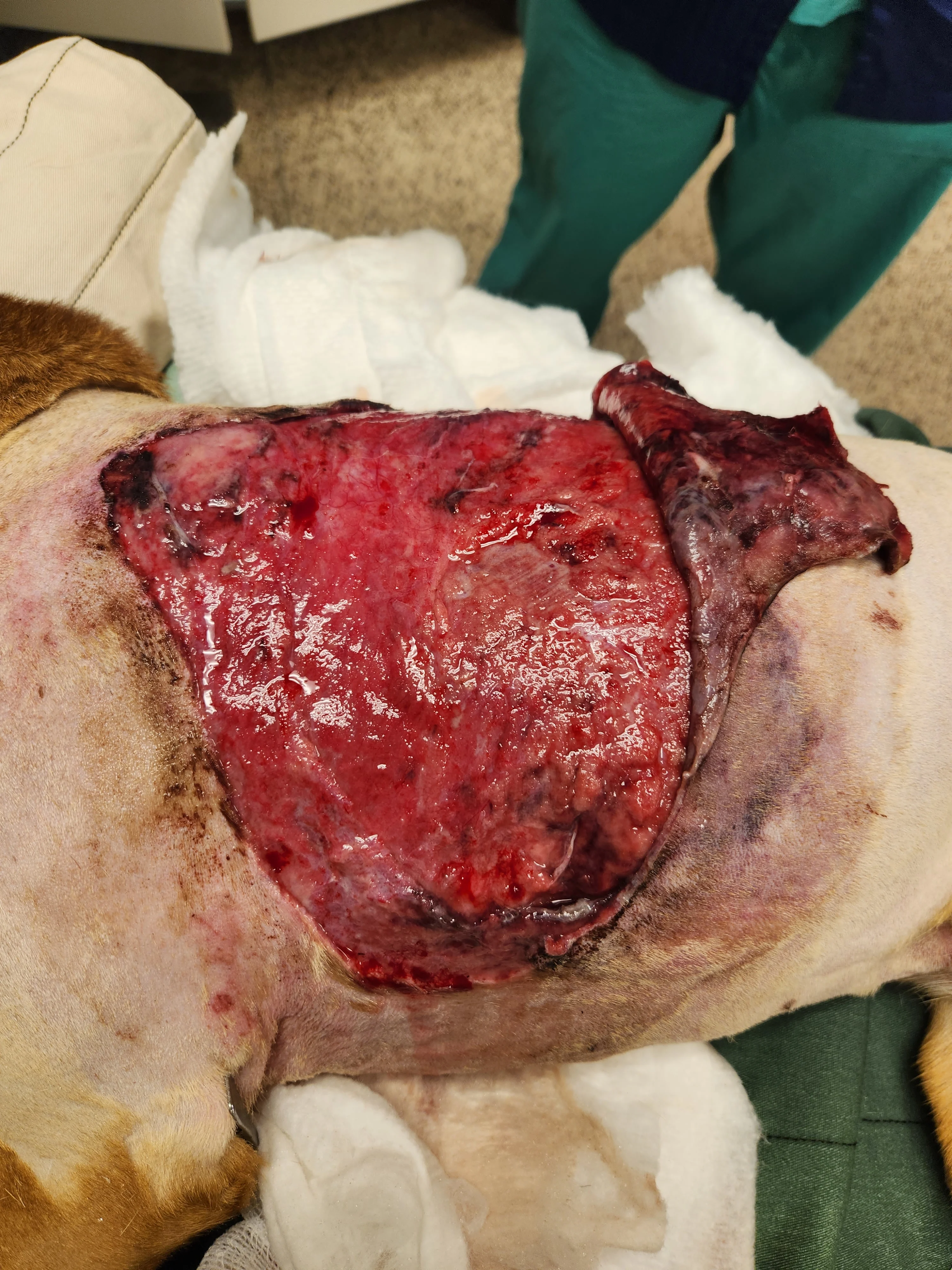
Figure 6 Over the next 3 days, the epithelium and underlying wound bed continued to develop new areas of necrosis daily, and substantial pocketing was noted caudally under the skin. With the patient under anesthesia, sharp surgical debridement was performed daily to remove necrotic tissues. Topical management included a 5-minute wound bed soak with 0.012% hypochlorous acid followed by application of medical-grade manuka honey gel, laparotomy pads, and a protective bandage.
Some topical antiseptics (eg, polyhexanide, hypochlorous acid) have broad antimicrobial activity without host cytotoxicity or documented resistance.1,15,16 Medical-grade manuka honey gel is antibacterial and anti-inflammatory and promotes wound healing.1 Absorptive bandages should be placed over honey dressings and may require daily changing because the hypertonicity of honey draws fluid from wounds.
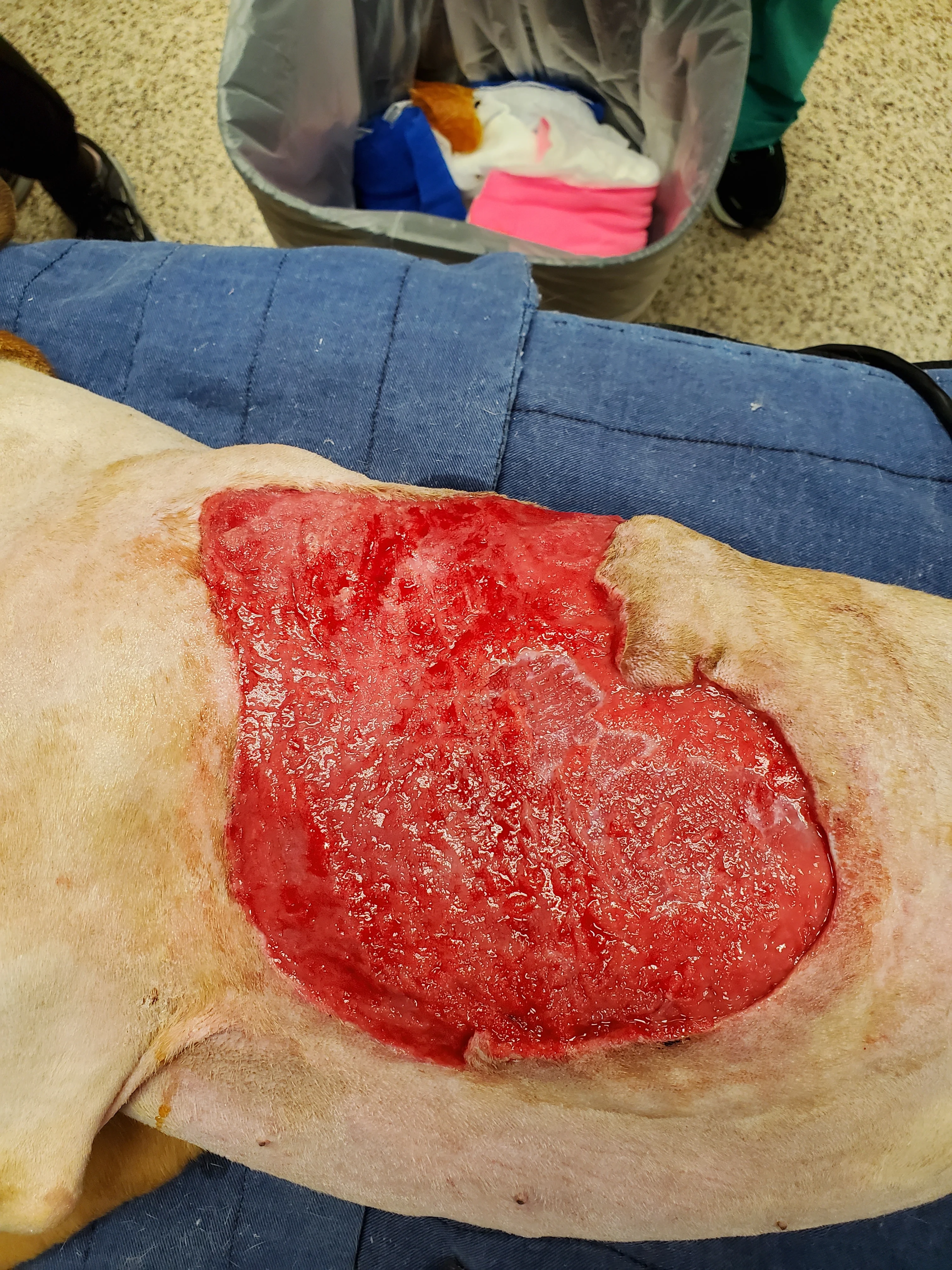
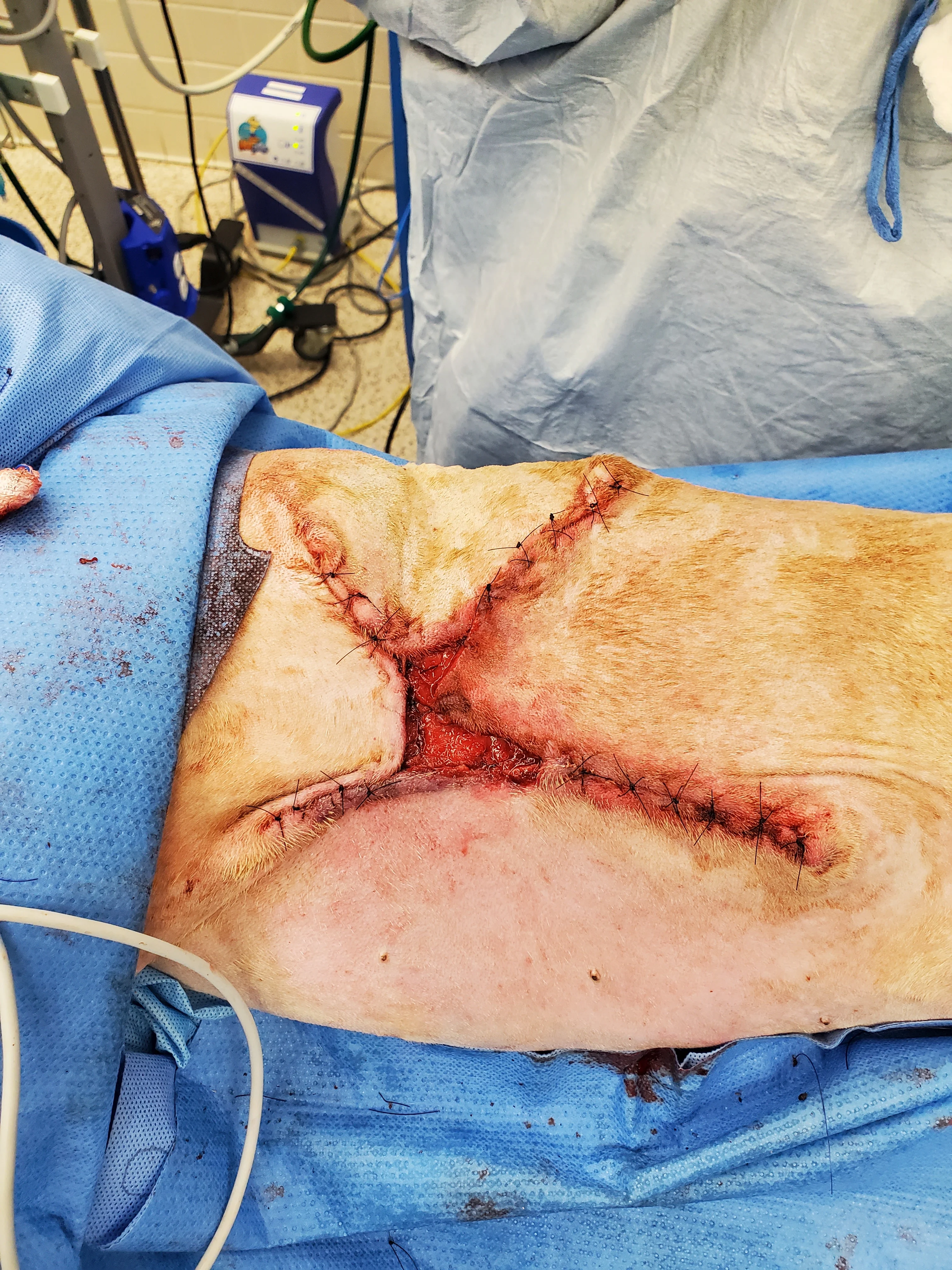
Figure 7 Seven days after presentation, the wound started filling with granulation tissue, and there was no further appearance of necrotic tissue (A). Surgery for partial wound closure was performed, with the skin elevated and undermined around the wound bed and advanced toward the center (B). Free edges were tacked down to the underlying wound bed. Walking sutures and subcutaneous tacking sutures were not used to avoid damaging the remaining blood supply.17 The central open wound was covered with a silver-impregnated, soft silicone foam dressing and a soft padded bandage.
Surgical closure of large, traumatic wounds should be delayed until the surrounding skin appears normal and contusions, edema, and infection have resolved.17 Once the wound bed is healthy, local and regional skin conditions should be considered to select a closure technique. In this patient, the health of nearby vascular pedicles was a concern because of the original skin avulsion and degloving; therefore, immediate complete closure and axial pattern and advancement flaps were not used. Instead, local tissues were undermined to release their attachments and maximize their elastic potential without overstretching the skin.17 Large wounds may close with less tension if they are envisioned as a triangle or rectangle and sutured from the corners inward in a Y- or X-shape.18

Figure 8 Two weeks after partial wound closure, skin along the closure sites was healed, and the central wound was filled with granulation tissue. The soft padded bandage and silver foam dressing were changed twice weekly for 2 additional weeks. The owners were then instructed to perform daily incisional care, consisting of applying a thin layer of topical neomycin, polymyxin B, bacitracin ointment to any remaining granulation tissue, covering the site with a nonadherent pad, and placing the dog in a clean t-shirt. The petrolatum base of the ointment helps provide moisture retention and facilitate epithelialization but may slow contraction.2 Four weeks later, the site was fully healed.
A variety of dressings are available for long-term open wound management. Sustained-release silver dressings are antimicrobial and often incorporated into absorbent, hydrophilic foam pads that may include alginates, dextrans, or other products. These dressings can be used for early granulating wounds, which require bandage changes every 3 to 4 days.3 The foam pad should be premoistened if the wound bed is dry.1 For fully granulated wounds, the combination of topical wound ointments or liquid foams with a nonadherent cotton dressing produces a semiocclusive dressing that can retain enough moisture to prevent dessication.2 A finely woven, lint-free shirt or body suit can help hold the dressing in place. This bandaging technique is inexpensive, easy, and safe and can allow owners to manage healthy, healing wounds in dogs and cats in the home.
Case 3: Incisional Discharge
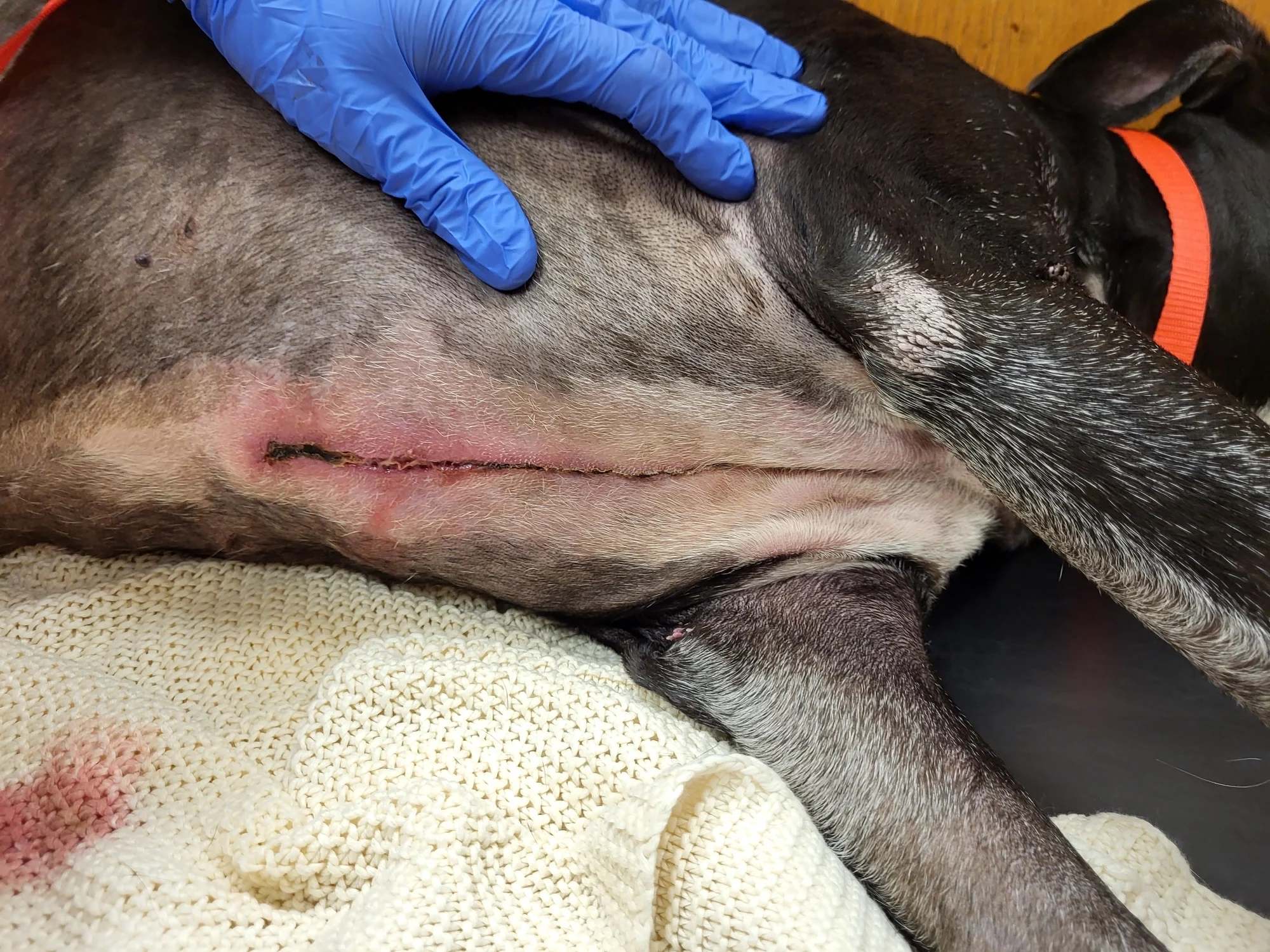
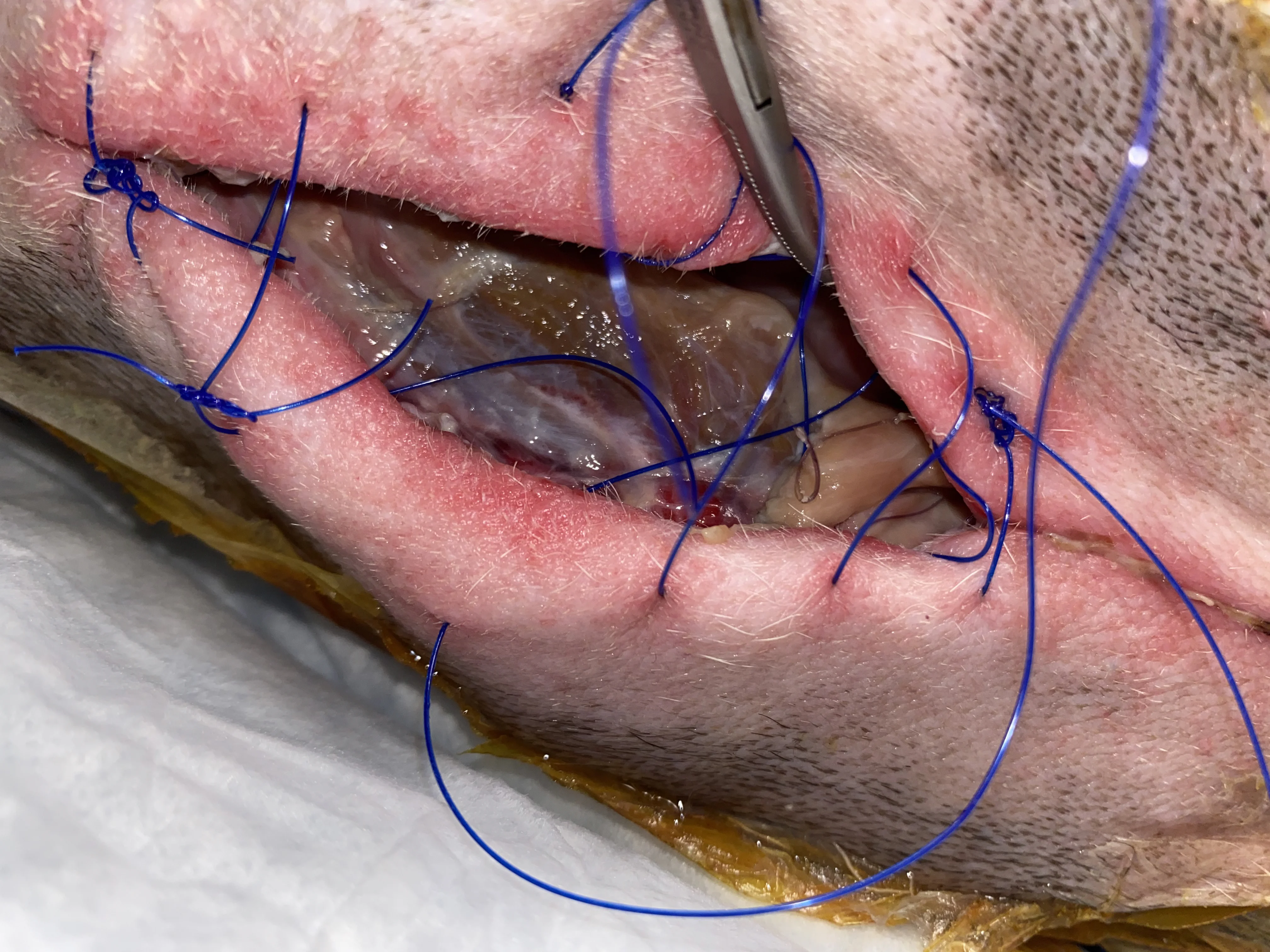
Figure 9 An obese (BCS, 9/9), 10-year-old neutered male American Staffordshire terrier was presented for incisional discharge 18 days after median sternotomy for thymoma removal, during which the sternebrae were reapposed with 0 and #1 polydioxanone suture in a figure-of-eight pattern. On presentation, serosanguinous fluid, cellulitis, and partial dehiscence were noted along the caudal extent of the incision. Thoracic radiographs and ultrasound were consistent with postoperative thoracotomy. The patient was anesthetized; fur around the wound was clipped; the wound was cleaned and surgically debrided; and tissue was collected for bacterial culture and susceptibility testing. A wide, loose continuous suture pattern of 0 polypropylene suture was placed in the skin, and a polyhexamethylene biguanide-impregnated gauze covered with medical-grade honey gel was inserted under the sutures, which were gently tightened to hold the dressing in place. The area was covered with sterile laparotomy pads held in place with an adhesive iodine-impregnated draping material. Ampicillin/sulbactam (30 mg/kg IV every 8 hours) was administered with the dog under anesthesia and continued overnight.
Closure-related complications of median sternotomy (eg, dehiscence, seroma, lameness, drainage, surgical site infections) are reported in 14% of dogs, with no significant difference between wire and suture closure, regardless of patient size.19

Figure 10 The wound was examined the next day with the patient under anesthesia. Cellulitis was still apparent; underlying tissues were discolored; and some sternal sutures were visible. Culture results subsequently indicated Serratia marcescens susceptible to marbofloxacin, doxycycline, trimethoprim sulfa, aminoglycosides, and chloramphenicol and methicillin-susceptible Staphylococcus aureus resistant to fluoroquinolones. The source of infection was thought to be from the dog’s skin, with S aureus likely transferred from the owner.
Incisional infections that occur within 30 days after soft tissue surgery are considered surgical site infections.20 Surgical site infections occur in 3% to 8.7% of dogs undergoing soft tissue surgery and 2.7% of dogs undergoing median sternotomy.19,20 Principles of surgical site infection management include opening the incision, debriding tissues, ascertaining whether body cavities are involved, addressing ongoing sources of contamination, obtaining a tissue culture, removing implants that are not functionally important, and using open wound management until the infection is cleared.20
S marcescens is an opportunistic pathogen in humans and can cause infection in dogs (rare), most commonly associated with bones, joints, or ears.21 Resistance to penicillin, potentiated penicillin, cephalosporins, macrolides, and tetracyclines is common. S marcescens can grow in 1% chlorhexidine solution.21
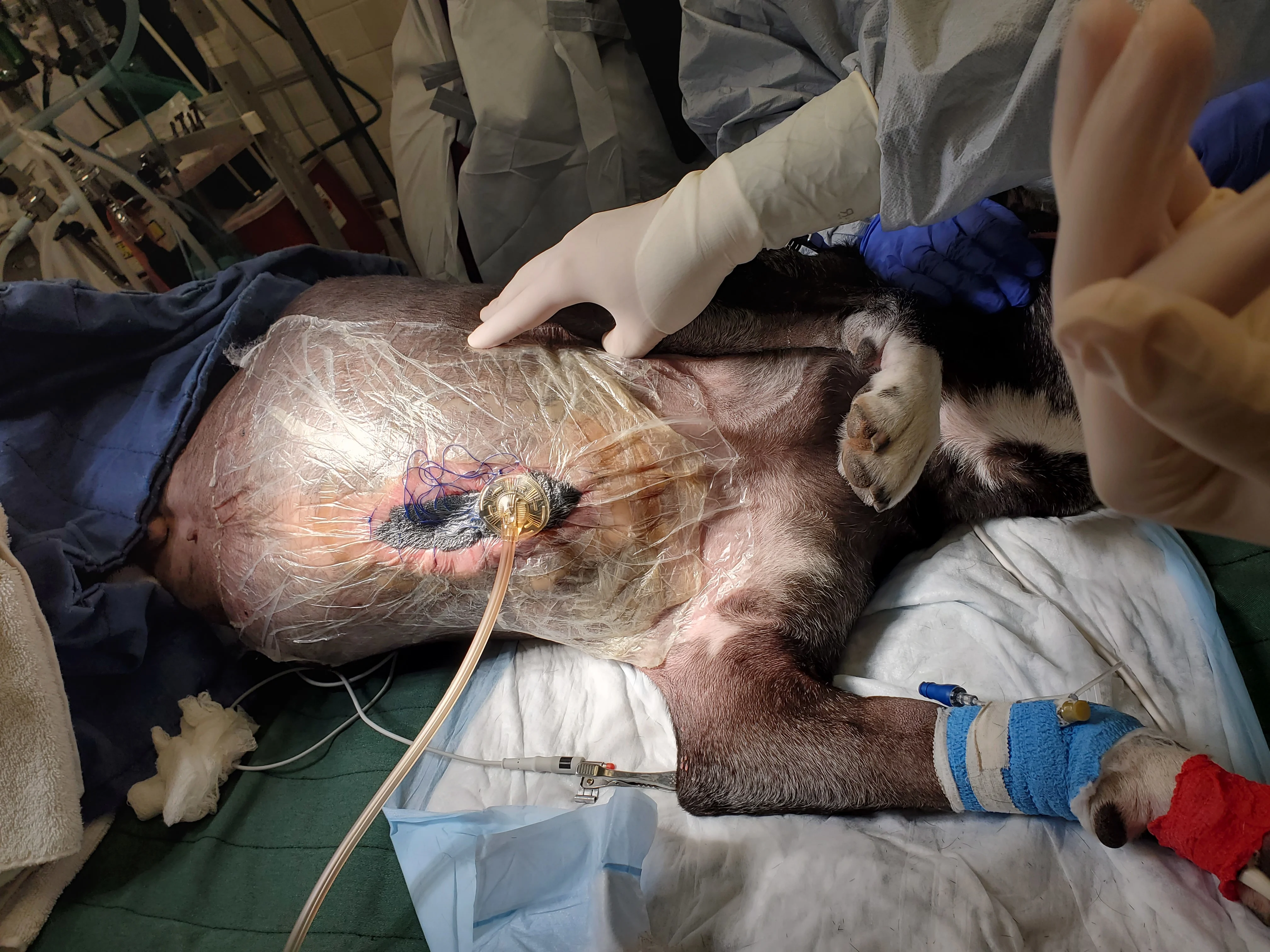
Figure 11 Negative pressure wound therapy (NPWT) was applied because of the wound location. Wounds treated with NPWT close more quickly than those treated with other traditional modalities. In this patient, NPWT would also maintain negative thoracic pressure if the sternal closure failed.22 Antibiotics were changed to chloramphenicol (47.5 mg/kg PO every 8 hours) based on in vitro susceptibilities and clinical effectiveness of this antimicrobial.23 In the authors’ experience, wounds infected with resistant Staphylococcus spp typically respond to systemic aminoglycosides or chloramphenicol in the veterinary setting.
Chloramphenicol is a broad-spectrum, bacteriostatic antimicrobial often used to treat methicillin-resistant Staphylococcus infections.24 Common adverse effects in dogs include GI upset, lethargy, restlessness, trembling, and increased liver enzymes.24 Large doses or prolonged administration may cause bone marrow suppression or peripheral neuropathy.24 Owners should use appropriate PPE when administering this drug, as there is risk for aplastic anemia in humans.24
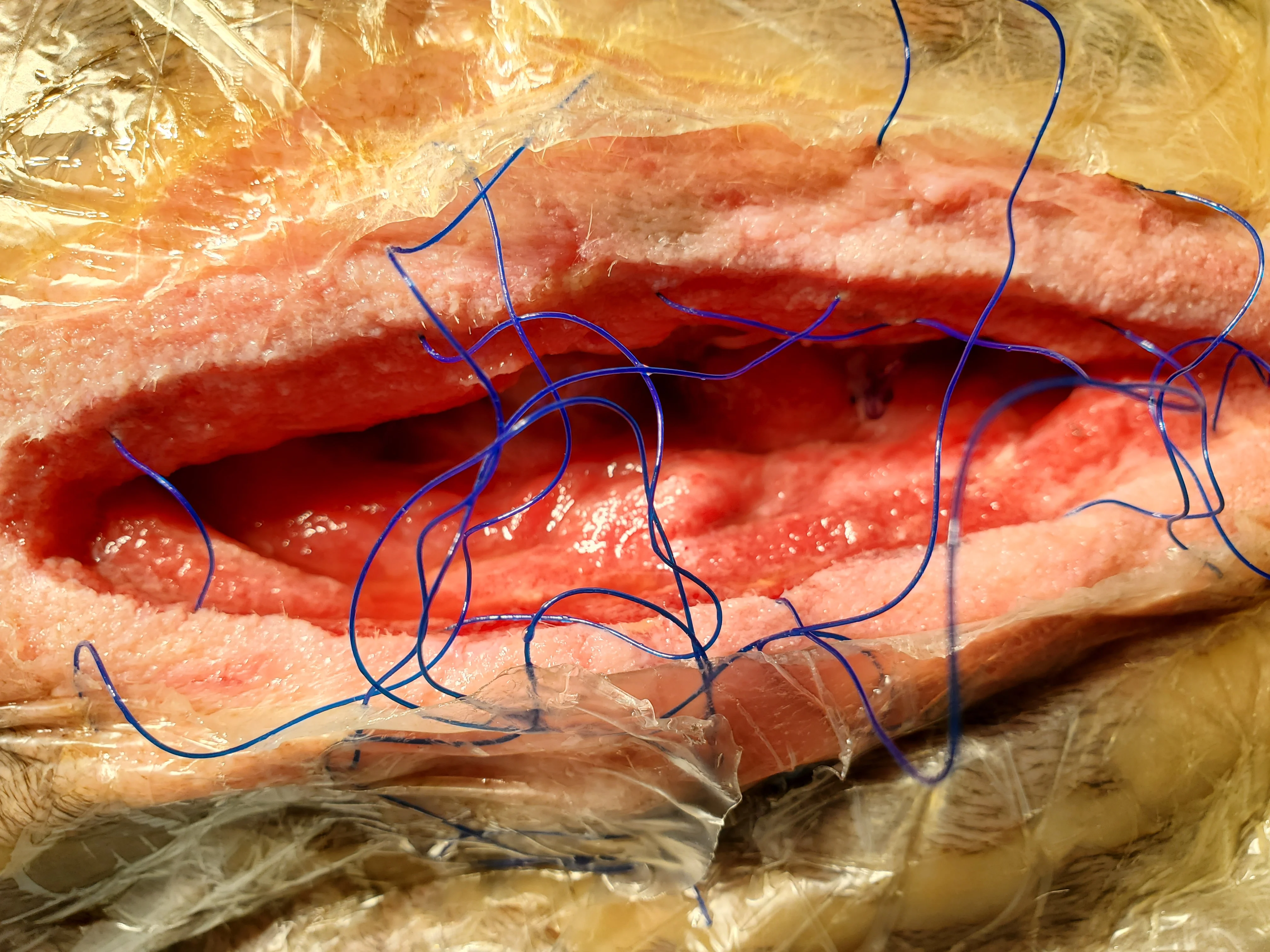
Figure 12 With the patient under anesthesia, the foam sponge was removed and the wound inspected every 2 to 3 days. Longer application of NPWT may result in granulation tissue ingrowth into the foam block.22 After 4 days of NPWT and antibiotic administration, cellulitis resolved, granulation tissue started to form near the wound margins, and a tissue culture was negative for S aureus but had a moderate growth of S marcescens with good susceptibility to trimethoprim sulfa but only intermediate susceptibility to chloramphenicol. Antimicrobial treatment was switched to trimethoprim sulfa (12.9 mg/kg PO every 12 hours).
In dogs with nonhealing wounds or infections from bacteria with susceptibility profiles that change over time, repeat cultures can help guide antimicrobial therapy.
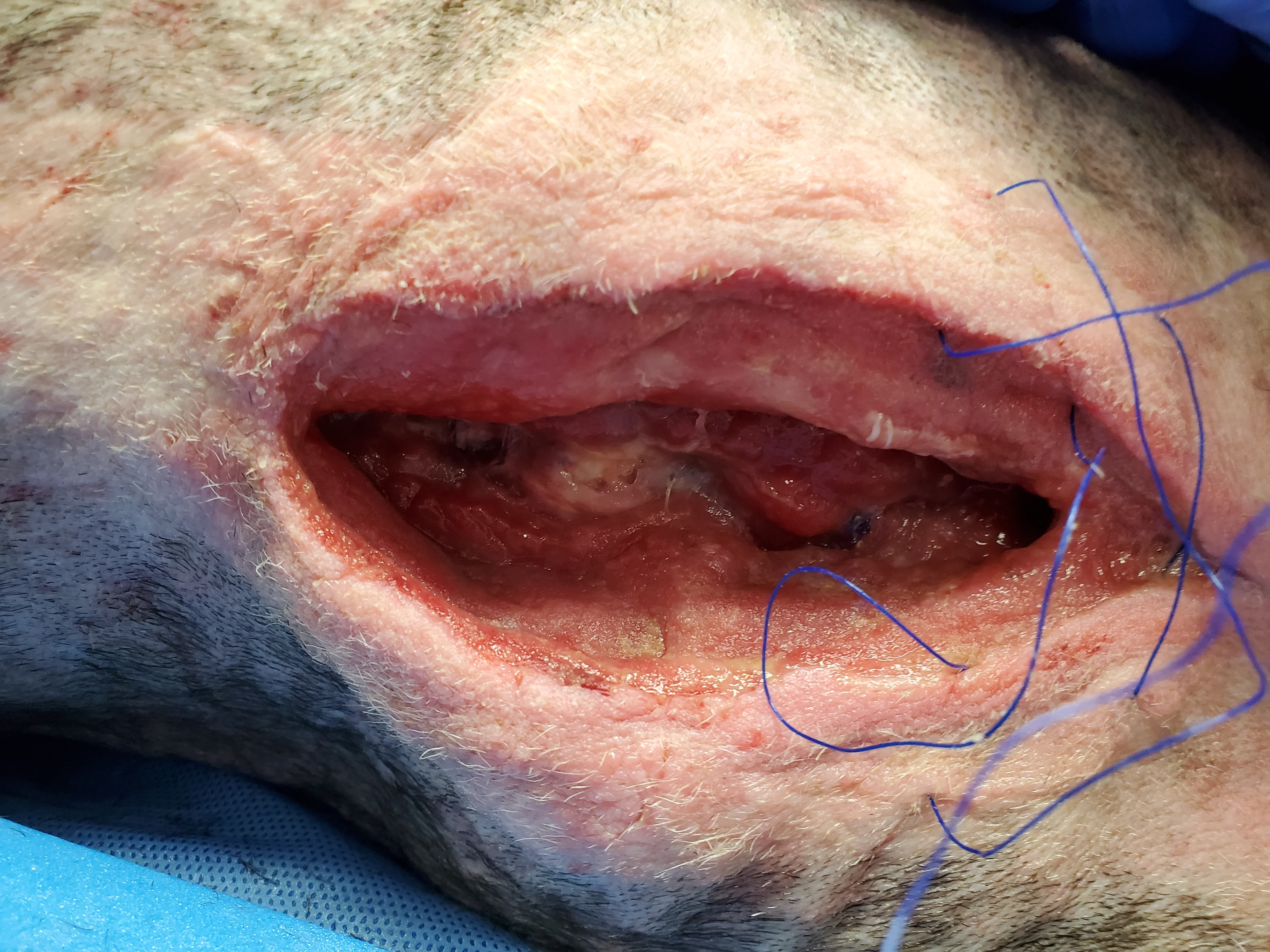
Figure 13 NPWT therapy should be continued until the wound is filled with granulation tissue. The wound can then undergo primary closure. Despite continued NPWT for 12 days, the wound in this patient became static, with minimal progression of granulation tissue development. A tissue culture taken after 9 days of NPWT was negative.
Failure of NPWT most commonly occurs if suction is lost.22 In addition, granulation tissue may not fill sites in which the foam sponge does not come into contact with subcutaneous tissues. Lack of response to NPWT is unusual but can occur with insufficient debridement.22 Other causes of delayed wound healing include persistent infection, biofilm formation, administration of immunosuppressive drugs, certain metabolic diseases (eg, uremia, hyperadrenocorticism, hypothyroidism), malnutrition, pressure, and radiation.25 In this dog, sternal osteomyelitis could have contributed to persistent infection, and additional radiographs may have been helpful in assessing the tissues.
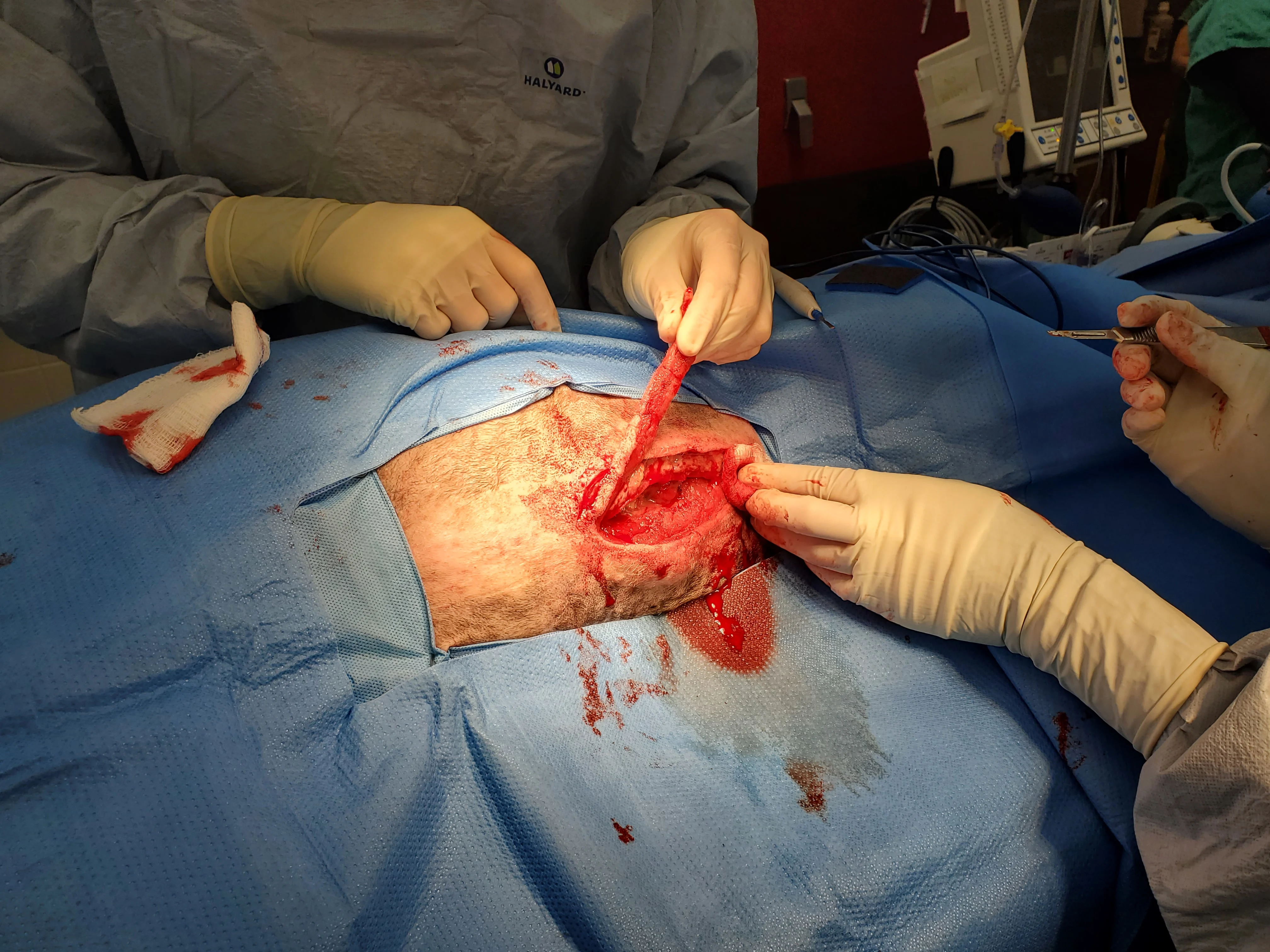
Figure 14 Due to the patient’s obesity and lack of apparent vascularity along the incision line, the lateral margins of the open wound were surgically resected until bleeding was seen. Obesity is a risk factor for wound complications in humans and was likely a factor in this patient.26 On histologic evaluation, resected material consisted of adipose tissue surrounded by dense fibrous connective tissue with moderate, multifocal granulomatous steatitis.
Adipose tissue has poor vascularity, increased fibrosis and tissue rigidity, and poor oxygen perfusion compared with other soft tissues and is more susceptible to mechanical damage.26 Resection of partially or fully granulated wounds can convert chronic or contaminated wounds into freshly incised wounds that can undergo immediate primary closure.4
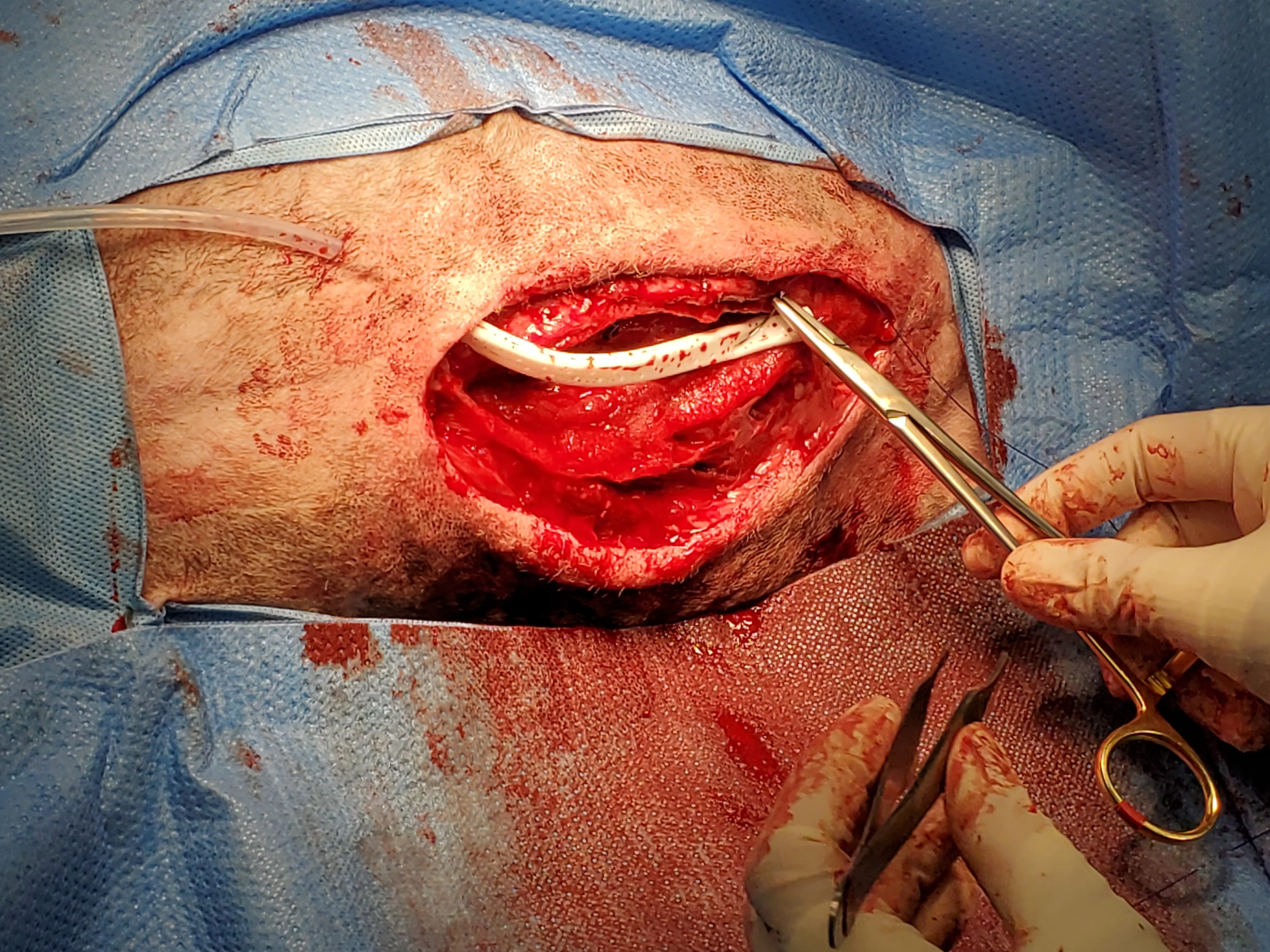
Figure 15 Dead space was expected, and tissues were thus closed over a continuous suction drain, which was removed after 3 days. The wound subsequently healed without complication.
Continuous suction drains actively remove exudate into a closed collection system, which lowers the risk for ascending infection compared with passive drains.27 The skin is protected, and bandage changes are not required because the exudate is contained; however, drain exit sites should remain covered to decrease the risk for ascending infection.2 Presence of the drain can cause inflammation; therefore, fluid production should be expected unless the drain becomes obstructed or loses suction. The drain is usually removed once fluid becomes serosanguinous and reduced in volume.27 Dogs are more likely to develop seromas after continuous suction drain removal if fluid production is >0.2 mL/kg/hour.28